Abstract
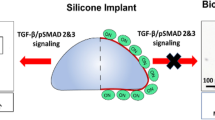
Capsular contracture remains a major complication after reconstructive or aesthetic breast augmentation. Formation of capsular fibrosis is a multifactorial process. An initial inflammatory reaction appears to be key to the development of capsular contracture. Recent studies have shown that pulsed acoustic cellular expression (PACE) has significant antiinflammatory effects. Thus, this study aimed to determine the potential of PACE to prevent or attenuate capsular contracture around silicone implants in a rodent model. For this study, 36 Lewis rats were divided into two groups, and a textured silicone implant was placed in a dorsal submuscular pocket. One group received PACE treatment, whereas the other group served as the control group and received no treatment. Follow-up evaluations were performed after 10, 35, and 100 days. Capsule thickness, collagen density, myofibroblasts, vascular density, and a semiquantitative real-time polymerase chain reaction that addressed differential gene expression were assessed. The PACE treatment significantly reduced capsule thickness on days 10, 35, and 100 compared with the control group (day 10: 632.9 ± 164.5 vs 932.6 ± 160.8, p < 0.05; day 35: 709.5 ± 175 vs 825.9 ± 313.3, p < 0.0.5; day 100: 736.3 ± 198.1 vs 1,062.3 ± 151.9, p < 0.05). This was accompanied by a significant suppression of proinflammatory genes (cluster of differentiation 68, monocyte chemotactic protein-1, CCL4) and synergistic alterations of pro- and antifibrotic proteins (transforming growth factor-beta 1, matrix metalloproteinase-2). This study showed that the PACE application significantly reduces capsular contracture around silicone implants. A decrease in capsular thickness after PACE treatment seems to be associated with a downregulation of proinflammatory genes and proteins. The study identifies PACE technology as a potential low-cost technique that is easy to use for reduction of capsular contracture after augmentation using silicone implants.
No Level Assigned
This journal requires that authors assign a level of evidence to each submission to which Evidence-Based Medicine rankings are applicable. This excludes Review Articles, Book Reviews, and manuscripts that concern Basic Science, Animal Studies, Cadaver Studies, and Experimental Studies. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors http://www.springer.com/00266.







Similar content being viewed by others
References
Stutman RL, Codner M, Mahoney A, Amei A (2012) Comparison of breast augmentation incisions and common complications. Aesthetic Plast Surg 36:1096–1104
Adams WP Jr (2009) Capsular contracture: What is it? What causes it? How can it be prevented and managed? Clin Plast Surg 36:119–126 vii
Araco A, Caruso R, Araco F, Overton J, Gravante G (2009) Capsular contractures: a systematic review. Plast Reconstr Surg 124:1808–1819
Heden P, Bronz G, Elberg JJ, Deraemaecker R, Murphy DK, Slicton A, Brenner RJ, Svarvar C, van Tetering J, van der Weij LP (2009) Long-term safety and effectiveness of style 410 highly cohesive silicone breast implants. Aesthetic Plast Surg 33:430–436 discussion 437–438
Cunningham B, McCue J (2009) Safety and effectiveness of Mentor’s MemoryGel implants at 6 years. Aesthetic Plast Surg 33:440–444
Spear SL, Murphy DK, Slicton A, Walker PS (2007) Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg 120(7 Suppl 1):8S–16S discussion 17S–18S
Bastos EM, Neto MS, Alves MT, Garcia EB, Santos RA, Heink T, Pereira JB, Ferreira LM (2007) Histologic analysis of zafirlukast’s effect on capsule formation around silicone implants. Aesthetic Plast Surg 31:559–565
Unlu RE, Yilmaz AD, Orbay H, Can B, Tekdemir I, Sensoz O (2007) Influence of rifampin on capsule formation around silicone implants in a rat model. Aesthetic Plast Surg 31:358–364
Lew DH, Yoon JH, Hong JW, Tark KC (2010) Efficacy of anti-adhesion barrier solution on periimplant capsule formation in a white rat model. Ann Plast Surg 65:254–258
Zeplin PH, Larena-Avellaneda A, Jordan M, Laske M, Schmidt K (2010) Phosphorylcholine-coated silicone implants: effect on inflammatory response and fibrous capsule formation. Ann Plast Surg 65:560–564
Zimman OA, Toblli J, Stella I, Ferder M, Ferder L, Inserra F (2007) The effects of angiotensin-converting-enzyme inhibitors on the fibrous envelope around mammary implants. Plast Reconstr Surg 120:2025–2033
Moreira M, Fagundes DJ, de Jesus Simoes M, Taha MO, Perez LM, Bazotte RB (2010) The effect of liposome-delivered prednisolone on collagen density, myofibroblasts, and fibrous capsule thickness around silicone breast implants in rats. Wound Repair Regen 18:417–425
Eisenberger F, Chaussy C (1978) Contact-free renal stone fragmentation with shock waves. Urol Res 6:111
Ogden JA, Toth-Kischkat A, Schultheiss R (2001) Principles of shock wave therapy. Clin Orthop Relat Res 387:8–17
Wang CJ (2003) An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J 26:220–232
Krokowicz L, Cwykiel J, Klimczak A, Mielniczuk M, Siemionow M (2010) Pulsed acoustic cellular treatment induces expression of proangiogenic factors and chemokines in muscle flaps. J Trauma 69:1448–1456
Kuo YR, Wu WS, Hsieh YL, Wang FS, Wang CT, Chiang YC, Wang CJ (2007) Extracorporeal shock wave-enhanced extended skin flap tissue survival via increase of topical blood perfusion and associated with suppression of tissue pro-inflammation. J Surg Res 143:385–392
Davis TA, Stojadinovic A, Anam K, Amare M, Naik S, Peoples GE, Tadaki D, Elster EA (2009) Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int Wound J 6:11–21
Krokowicz L, Klimczak A, Cwykiel J, Mielniczuk M, Grykien C, Siemionow M (2012) Pulsed acoustic cellular expression as a protective therapy against I/R injury in a cremaster muscle flap model. Microvasc Res 83:213–222
Reichenberger MA, Heimer S, Schaefer A, Lass U, Gebhard MM, Germann G, Engel H, Kollensperger E, Leimer U, Mueller W (2012) Extracorporeal shock wave treatment protects skin flaps against ischemia-reperfusion injury. Injury 43:374–380
Turabelidze A, Guo S, DiPietro LA (2010) Importance of housekeeping gene selection for accurate reverse transcription-quantitative polymerase chain reaction in a wound healing model. Wound Repair Regen 18:460–466
Anderson JM, Rodriguez A, Chang DT (2008) Foreign body reaction to biomaterials. Semin Immunol 20:86–100
Puskas JE, Luebbers MT (2012) Breast implants: the good, the bad and the ugly: can nanotechnology improve implants? Wiley Interdiscip Rev Nanomed Nanobiotechnol 4:153–168
Yan X, Zeng B, Chai Y, Luo C, Li X (2008) Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann Plast Surg 61:646–653
Ghaly A, Marsh DR (2010) Ischaemia-reperfusion modulates inflammation and fibrosis of skeletal muscle after contusion injury. Int J Exp Pathol 91:244–255
Schwartzkopff F, Petersen F, Grimm TA, Brandt E (2012) CXC chemokine ligand 4 (CXCL4) down-regulates CC chemokine receptor expression on human monocytes. Innate Immun 18(1):124–139
Chung AS, Kao WJ (2009) Fibroblasts regulate monocyte response to ECM-derived matrix: the effects on monocyte adhesion and the production of inflammatory, matrix remodeling, and growth factor proteins. J Biomed Mater Res A 89:841–853
Gharaee-Kermani M, Denholm EM, Phan SH (1996) Costimulation of fibroblast collagen and transforming growth factor-beta 1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem 271:17779–17784
Hara A, Sakai N, Furuichi K, Sakai Y, Takeya M, Bucala R, Mukaida N, Takuwa Y, Matsushima K, Kaneko S, Wada T (2013) CCL2/CCR2 augments the production of transforming growth factor-beta 1, type 1 collagen, and CCL2 by human CD45-/collagen 1-positive cells under high glucose concentrations. Clin Exp Nephrol. doi:10.1007/s10157-013-0796-6
Ruiz-de-Erenchun R, de las Dotor Herrerias J, Hontanilla B (2005) Use of the transforming growth factor-beta1 inhibitor peptide in periprosthetic capsular fibrosis: experimental model with tetraglycerol dipalmitate. Plast Reconstr Surg 116:1370–1378
Tamboto H, Vickery K, Deva AK (2010) Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg 126:835–842
Acknowledgments
The dermaPACE device was kindly provided by Sanuwave, Inc. (Alpharetta, GA, USA). The silicone implants were kindly provided by Pascal Hüser from Polytech Health and Aesthetic (Dieburg, Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reichenberger, M.A., Heimer, S., Lass, U. et al. Pulsed Acoustic Cellular Expression (PACE) Reduces Capsule Formation Around Silicone Implants. Aesth Plast Surg 38, 244–251 (2014). https://doi.org/10.1007/s00266-013-0235-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-013-0235-9