Abstract
Background
Polyacrylamide hydrogel, considered a safe and biocompatible soft tissue filler, is widely used in cosmetic procedures. Its use for facial contouring and breast augmentation in Iran has increased dramatically in recent years. Most patients and many doctors are unaware of possible and reported adverse effects related to its administration.
Methods
This study enrolled 98 patients experiencing unsatisfactory results and complications of polyacrylamide hydrogel. Adverse effects related to gel administration were documented for all the patients. Lab values were requested together with related medical care and surgical treatments, and gel was extracted by incision, milking, and irrigation.
Results
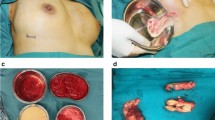
The most common findings at the time of presentation were inflammation (n = 51), asymmetry (n = 31), irregularity (n = 18), infection and abscess formation (n = 11), and gel migration (n = 8). In one patient, severe anaphylactoid reaction was observed 1 week after gel injection, which led to significant complications for the patient. Histologic findings showed granuloma formation (n = 17), fat necrosis (n = 9), and fibrosis (n = 17). Macroscopic gel-related complications resolved after extraction of the injected material, except for skin necrosis and hyperpigmentation, which remained unchanged. For eight patients, the gel could not be extracted by squeezing and irrigation entirely. Three patients experienced gel reaccumulation after seemingly complete removal of the gel.
Conclusions
A wide range of complications seen among our patients showed that polyacrylamide hydrogel may not be as safe and biocompatible as it was thought previously. Both patients and physicians must be aware of the potential side effects of polyacrylamide hydrogel before gel administration.




















Similar content being viewed by others
References
McCollister DD, Hake CL, Sadek SE, Rowe VK (1965) Toxicologic investigations of polyacrylamides. Toxicol Appl Pharmacol 7:639–651
Smith EA, Oehme FW (1991) Acrylamide and polyacrylamide: a review of production, use, environmental fate, and neurotoxicity. Rev Environ Health 9:215–228
Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int J Toxicol 24(2):21–50 (2005)
Breiting V, Aasted A, Jorgensen A, Opitz P, Rosetzsky A (2004) A study on patients treated with polyacrylamide hydrogel injection for facial corrections. Aesth Plast Surg 28:45–53
Zarini E, Supino R, Pratesi G, Laccabue D, Tortoreto M, Scanziani E, Ghisleni G, Paltrinieri S, Tunesi G, Nava M (2004) Biocompatibility and tissue interactions of a new filler material for medical use. Plast Reconstr Surg 114:934–942
de Cassia Novaes W, Berg A (2003) Experiences with a new nonbiodegradable hydrogel (Aquamid): a pilot study. Aesth Plast Surg 27:376–380
Christensen LH, Breiting VB, Aasted A, Jorgensen A, Kebuladze I (2003) Long-term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr Surg 111:1883–1890
Christensen L, Breiting V, Janssen M, Vuust J, Hogdall E (2005) Adverse reactions to injectable soft tissue permanent fillers. Aesth Plast Surg 29:34–48
Niechajev I (2000) Lip enhancement: surgical alternatives and histologic aspects. Plast Reconstr Surg 105:1173–1183 (discussion 1184–1177)
Bello G, Jackson IT, Keskin M, Kelly C, Dajani K, Studinger R, Kim EM, Lincoln D, Silberberg B, Lee A (2007) The use of polyacrylamide gel in soft tissue augmentation: an experimental assessment. Plast Reconstr Surg 119:1326–1336
De Santis G, Jacob V, Baccarani A, Pedone A, Pinelli M, Spaggiari A, Guaraldi G (2008) Polyacrylamide hydrogel injection in the management of human immunodeficiency virus-related facial lipoatrophy: a 2-year clinical experience. Plast Reconstr Surg 121:644–653
von Buelow S, Pallua N (2006) Efficacy and safety of polyacrylamide hydrogel for facial soft-tissue augmentation in a 2-year follow-up: a prospective multicenter study for evaluation of safety and aesthetic results in 101 patients. Plast Reconstr Surg 118:85S–91S
Zhao Y, Qiao Q, Yue Y, Kou X, Liu Z (2004) Clinical and histologic evaluation of a new injectable implant: hydrophilic polyacrylamide gel. Ann Plast Surg 53:267–272
Goldan O, Georgiou I, Grabov-Nardini G, Regev E, Tessone A, Liran A, Haik J, Mendes D, Orenstein A, Winkler E (2007) Early and late complications after a nonabsorbable hydrogel polymer injection: a series of 14 patients and novel management. Dermatol Surg 33(Suppl 2):S199–S206 (discussion S206)
de Bree R, Middelweerd MJ, van der Waal I (2004) Severe granulomatous inflammatory response induced by injection of polyacrylamide gel into the facial tissue. Arch Facial Plast Surg 6:204–206
El-Shafey El-SI (2008) Complications from repeated injection or puncture of old polyacrylamide gel implant sites: case reports. Aesth Plast Surg 32:162–165
Christensen L, Breiting V (2005) Complications induced by polyacrylamide hydrogen injection. Plast Reconstr Surg 116:1168–1169
Christensen L, Breiting V (2006) Management of postoperative complications of breast augmentation by injected polyacrylamide hydrogel. Aesth Plast Surg 30:132
Cohen JL (2008) Understanding, avoiding, and managing dermal filler complications. Dermatol Surg 34(Suppl 1):S92–S99
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manafi, A., Emami, AH., Pooli, A.H. et al. Unacceptable Results with an Accepted Soft Tissue Filler: Polyacrylamide Hydrogel. Aesth Plast Surg 34, 413–422 (2010). https://doi.org/10.1007/s00266-009-9359-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-009-9359-3