Abstract
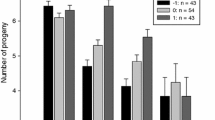
Numerous studies have examined the correlation between offspring quantity and quality, and many have found that the most common brood size is often smaller than broods with the highest offspring quality or production. However, the reasons why these small broods with lower offspring quality are produced are still poorly explained. Using data spanning 29 years, we investigated the effects of brood size on nestlings’ body mass and the lifetime fitness for those offspring as adults (as proxies of offspring quality) in the Crested Ibis (Nipponia nippon). We also examined the temporal variation of brood size. We found that overall offspring quality increases with brood size and that individuals from broods of three had the highest quality, as quantified by larger body mass, higher adult survival, and lifetime reproductive success. Furthermore, the brood size of an individual pair significantly varied across years, and the proportion of broods containing two offspring increased while broods of three decreased after 2000 when the population dispersed to low-quality habitat. These findings indicate that spatiotemporal variation in resources may impact variation in brood size and subsequent fitness consequences, and that small broods are more common in resource-poor years or low-quality habitats. In contrast, parents with access to high-quality resources produce larger broods of nestlings that achieve higher body mass and subsequently experience higher adult survival and lifetime fitness. This study highlights how variation in life history traits can be influenced by resource condition and provides an insight into particular habitats that need conservation for Crested Ibis.
Significance statement
Although life history theory predicts a trade-off between offspring quantity and quality, and that fewer, high-quality offspring are expected to be more common to prolong one’s own survival prospect in long-lived species, birds, mammals, and humans often show a positive correlation for these traits. Why do parents produce small broods with lower offspring quality? Here, we found that offspring quality—such as nestlings’ body mass, survival, and the lifetime reproductive success of offspring as adults—increased overall with brood size, up to broods of three of Crested Ibis. Brood size varied across years; in particular, pairs appear to produce smaller broods of nestlings that have lower body mass and lifetime fitness in resource-poor years or lower-quality habitats. This long-term study helps to advance our understanding of the fitness consequences and ecological mechanisms that impact offspring quantity and quality in long-lived animals.




Similar content being viewed by others
Data availability
The datasets used in this work are available in the Dryad Digital Repository, https://doi.org/10.5061/dryad.x95x69pjx.
References
Alberts SC (2019) Social influences on survival and reproduction: insights from a long-term study of wild baboons. J Anim Ecol 88:47–66
Bartoń K (2016) MuMIn: multi-model inference, R package version 1.42.1. https://CRAN.R-project.org/package=MuMIn/. Accessed 10 Nov 2022
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
BirdLife International (2001) Threatened birds of Asia: the BirdLife International Red Data Book. BirdLife International, Cambridge, UK, pp 315–329
Boag PJ, van Noordwijk AJ (1987) Quantitative genetics. In: Cooke F, Buckley PA (eds) Avian Genetics. Academic Press, London, pp 45–78
Bosman DS, Stienen E, Lens L (2016) Sex, growth rate, rank order after brood reduction, and hatching date affect first-year survival of long-lived herring gulls. J Field Ornithol 87:391–403
Boyce AJ, Freeman BG, Mitchell AE, Martin TE (2015) Clutch size declines with elevation in tropical birds. Auk 132:424–432
Brooks ME, Kristensen K, Darrigo MR, Rubim P, Uriarte M, Bruna E, Bolker BM (2019) Statistical modeling of patterns in annual reproductive rates. Ecol 100:e02706
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, Berlin
Calvert AM, Alisauskas RT, Kellett DK (2019) Fitness heterogeneity in adult snow and ross’s geese: survival is higher in females with brood patches. Auk 136:1–16
Charmantier A, Perrins C, Mccleery RH, Sheldon BC (2006) Evolutionary response to selection on clutch size in a long-term study of the mute swan. Am Nat 167:453–465
Delehanty DJ, Oring LW (1993) Effect of clutch size on incubation persistence in male Wilson’s phalaropes (Phalaropus tricolor). Auk 110:521–528
Ding C (2004) Research on the Crested Ibis. Shanghai Scientific and Technological Educational Publishing House, Shanghai, China
Ding C (2010) Crested Ibis Chinese Birds 1:156–162
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 80:225–252
Emery Thompson M, Muller MN, Sabbi K, Machanda ZP, Otali E, Wrangham RW (2016) Faster reproductive rates trade off against offspring growth in wild chimpanzees. P Natl Acad Sci USA 113:7780–7785
Fuirst M, Strickland D, Norris DR (2021) Breeding dispersal in a resident boreal passerine can lead to short- and long-term fitness benefits. Ecosphere 12:e03747
Gillespie DO, Russell AF, Lummaa V (2008) When fecundity does not equal fitness: evidence of an offspring quantity versus quality trade-off in pre-industrial humans. Proc R Soc Lond B 275:713–722
Hamel S, Côté SD, Gaillard JM, Festa-Bianchet M (2009) Individual variation in reproductive costs of reproduction: high-quality females always do better. J Anim Ecol 78:143–151
Hao X, Zou T, Han X, Zhang F, Du W (2021) Grow fast but don’t die young: maternal effects mediate life-history trade-offs of lizards under climate warming. J Anim Ecol 90:1550–1559
Harrison XA, Blount JD, Ryan Norris RD, Bearhop S (2011) Carry-over effects as drivers of fitness differences in animals. J Anim Ecol 80:4–18
Haywood S, Perrins CM (1992) Is clutch size in birds affected by environmental conditions during growth? Proc R Soc Lond B 249:195–197
He X, Qing B, Han J, Ding C (2013) Improved molecular assay for sex identification of the endangered crested ibis (Nipponia nippon) based on the CHD1 gene and a sex-linked microsatellite locus. Zool Sci 30:742–747
Herzog MP (2002) Environmental regulation of growth in black brant. PhD thesis, University of Alaska Fairbanks, Fairbanks, Alaska, USA
Huang Y, Ye Y, Zhang Y, Barras A, Wang C, Qing B, Ding C (2022) Tall trees drive the nest-site selection of wild Crested Ibis Nipponia nippon. Bird Conserv Int 32:486–497
Jacobsen KO, Erikstad KE, Saether BE (1995) An experimental study of the costs of reproduction in the kittiwake Rissa tridactyla. Ecol 76:1636–1642
Kowalczyk ND, Chiaradia A, Preston TJ, Reina RD (2014) Linking dietary shifts and reproductive failure in seabirds: a stable isotope approach. Funct Ecol 28:755–765
Laake JL (2013) RMark: An R Interface for analysis of capture-recapture data with MARK. AFSC Processed Rep.2013-01, 25 p. Alaska Fish. Sci. Cent., NOAA, Natl. Mar. Fish. Serv., 7600 Sand Point Way NE, Seattle WA 98115
Lack D (1947) The significance of clutch size. Ibis 89:302–352
Lack D (1967) The significance of clutch size in waterfowl. Wildfowl 18:125–128
Larsen VA, Lislevand T, Byrkjedal I (2003) Is clutch size limited by incubation ability in northern lapwings? J Anim Ecol 72:784–792
Larsson K, Forslund P (1991) Environmentally induced morphological variation in the barnacle goose, Branta leucopsis. J Evol Biol 4:619–636
Leach AG, Van Dellen AW, Riecke TV, Sedinger JS (2017) Incubation capacity contributes to constraints on maximal clutch size in Brent Geese Branta bernicla nigricans. Ibis 159:588–599
Leach AG, Sedinger JS, Riecke TV, Van Dellen AW, Ward DH, Boyd WS (2019) Brood size affects future reproduction in a long-lived bird with precocial young. Am Nat 193:458–471
Lengyel S (2007) Benefits of large broods by higher chick survival and better territories in a precocial shorebird. Behav Ecol Sociobiol 61:589–598
Lengyel S, Kiss B, Tracy CR (2009) Clutch size determination in shorebirds: revisiting incubation limitation in the pied avocet (Recurvirostra avosetta). J Anim Ecol 78:396–405
Lepage D, Gauthier G, Desrochers A (1998) Larger clutch size increases fledging success and offspring quality in a precocial species. J Anim Ecol 67:210–216
Lessells CM (1986) Brood size in Canada geese: a manipulation experiment. J Anim Ecol 55:669–689
Liu Y (1981) Rediscovery of Crested Ibis Nipponia nippon in Qinling Mountain. Chinese J Zool 27:237
Loonen M, Bruinzeel LW, Black JM, Drent RH (1999) The benefit of large broods in barnacle geese: a study using natural and experimental manipulations. J Anim Ecol 68:753–768
Madsen T, Shine R (2000) Silver spoons and snake body sizes: prey avail- ability early in life influences long-term growth rates of free-ranging pythons. J Anim Ecol 69:952–958
Magnusson A, Skaug H, Nielsen A, Berg C, Kristensen K, Maechler M, van Bentham K, Bolker B, Brooks M (2017) glmmTMB: generalized linear mixed models using a template model builder. https://cran.r-project.org/web/packages/glmmTMB/index.html. Accessed 10 Nov 2022
Martin TE (1996) Life history evolution in tropical & south temperate birds: what do we really know? J Avian Biol 27:263–272
Marvelde LT, Webber SL, Meijer H, Visser ME (2012) Energy expenditure during egg laying is equal for early and late breeding free-living female great tits. Oecologia 168:631–638
McNamara J, Barta Z, Wikelski M, Houston AI (2008) A theoretical investigation of the effect of latitude on avian life histories. Am Nat 172:331–345
Meyrier E, Jenni L, Bötsch Y, Strebel S, Erne B, Tablado Z (2017) Happy to breed in the city? Urban food resources limit reproductive output in Western Jackdaws. Ecol Evol 7:1363–1374
Millon A, Petty SJ, Little B, Lambin X (2011) Natal conditions alter age-specific reproduction but not survival or senescence in a long-lived bird of prey. J Anim Ecol 80:968–975
Minias P, Włodarczyk R, Surmacki A, Iciek T (2015) Silver spoon effects on plumage quality in a passerine bird. R Soc Open Sci 2:140459
Moore MP, Martin RA (2019) On the evolution of carry-over effects. J Anim Ecol 88:1832–1844
Mulder MB (2000) Optimizing offspring: the quantity-quality tradeoff in agropastoral Kipsigis. Evol Hum Behav 21:391–410
Nicolai CA, Sedinger JS (2012) Trade-offs between offspring fitness and future reproduction of adult female black brent. J Anim Ecol 81:798–805
Owen M, Black JM (1989) Factors affecting the survival of barnacle geese on migration from the breeding grounds. J Anim Ecol 58:603–617
Parejo D, Danchin E (2006) Brood size manipulation affects frequency of second clutches in the blue tit. Behav Ecol Sociobiol 60:184–194
Pettifor RA (1993) Brood manipulations experiments. I. The number of offspring surviving per nest in blue tits (Parus caeruleus). J Anim Ecol 62:131–144
Pettifor RA, Perrins CM, McCleery RH (2001) The individual optimization of fitness: variation in reproductive output, including clutch size, mean nestling mass and offspring recruitment, in manipulated broods of great tits Parus major. J Anim Ecol 70:62–79
Pichorim M, Monteiro-Filho E (2008) Brood size and its importance for nestling growth in the biscutate swift (Streptoprocne biscutata, Aves: Apodidae). Braz J Biol 68:851–857
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/. Accessed 10 Nov 2022
Ricklefs RE (2000) Density dependence, evolutionary optimization, and the diversification of avian life histories. Condor 102:9–22
Roff D, Heibo E, Vøllestad L (2006) The importance of growth and mortality costs in the evolution of the optimal life history. J Evol Biol 19:1920–1930
Rohwer FC (1992) The evolution of reproductive patterns in waterfowl. In: Batt BDJ, Afton AD, Anderson MG, Ankney CD, Johnson DH, Kadlec JA, Krapu GL (eds) Ecology and management of breeding water fowl. University of Minnesota Press, Minneapolis, pp 486–539
Roulin A, Ducrest AL, Dijkstra C (1999) Effect of brood size manipulations on parents and offspring in the barn owl Tyto alba. Ardea 87:91–100
Rushing CS, Marra PP, Dudash MR (2016) Winter habitat quality but not long-distance dispersal influences apparent reproductive success in a migratory bird. Ecol 97:1218–1227
Rytkönen S, Orell M (2001) Great tits, Parus major, lay too many eggs: experimental evidence in mid-boreal habitats. Oikos 93:439–450
Sæther BE (1988) Pattern of covariation between life-history traits of European birds. Nature 331:616–617
Sandercock BK (1997) Incubation capacity and clutch size determination in two calidrine sandpipers: a test of the four-egg threshold. Oecologia 110:50–59
Sedinger JS, Chelgren ND (2007) Survival and breeding advantages of larger Black Brant goslings: within and among cohort variation. Auk 124:1281–1293
Sedinger JS, Flint PL, Lindberg MS (1995) Environmental influence on life-history traits: growth, survival, and fecundity in Black Brant (Branta bernicla). Ecol 76:2404–2414
Sedinger JS, VanDellen AW, Leach AG, Riecke TV (2017) Ultimate regulation of fecundity in species with precocial young: declining marginal value of offspring with increasing brood size does not explain maximal clutch size in black brent geese. Oecologia 183:431–440
Sellers KF, Shmueli G (2010) A flexible regression model for count data. Ann Appl Stat 4:943–961
Sepp T, McGraw KJ, Kaasik A, Giraudeau M (2017) A review of urban impacts on avian life-history evolution: does city living lead to slower pace of life? Global Change Biol 24:1452–1469
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Smith RJ, Moore FR (2005) Arrival timing and seasonal reproductive performance in a long-distance migratory landbird. Behav Ecol Sociobiol 57:231–239
Song Z (2018) Sex allocation pattern and reproduction strategy in the wild population of Crested Ibis (Nipponia nippon). PhD thesis, Beijing Forestry University, Beijing, China
Song Z, Zou Y, Hu C, Ye Y, Wang C, Qing B, Komdeur J, Ding C (2019) Silver spoon effects of hatching order in an asynchronous hatching bird. Behav Ecol 30:509–517
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford, UK
van Noordwijk AJ, de Jong G (1986) Acquisition and allocation of resources: their influence on variation in life history tactics. Am Nat 128:137–142
Wang Z, Zhai T (2001) Reproduction of the wild Nipponia nippon in Yang County. Chinese J Ecol 20:12–15
Wang C, Liu D, Qing B, Ding H, Cui Y, Ye Y, Lu J, Yan L, Ke L, Ding C (2014) The current population and distribution of wild Crested Ibis Nipponia nippon. Chinese J Zool 49:666–671
Wang C, Zhang Y, Zeng J, Gao J, Yan L, Liu D (2020) Reproductive status and population size of wild Crested Ibis (Nipponia nippon) in China. Sci Silvae Sin 56:143–150
Weimerskirch H (2018) Linking demographic processes and foraging ecology in wandering albatross—conservation implications. J Anim Ecol 87:945–955
White GC, Burnham KP (1999) Program mark: survival estimation from populations of marked animals. Bird Study 46:120–139
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100:687–690
Xu X, Jiang J, Lei Y, Wang C, Qing B, Ding C (2022) Using stable isotope to compare the habitat use and trophic level between the new and old breeding range of wild Crested Ibis in the early breeding season. Avian Res 13:100007
Ye Y, Jiang Y, Hu C, Liu Y, Qing B, Wang C, Esteban FJ, Ding C (2017) What makes a tactile forager join mixed-species flocks? A case study with the endangered Crested Ibis (Nipponia nippon). Auk 134:421–431
Ye Y, Simone S, Song Z, Hu C, Zhang Z, Qing B, Wang C, Ding C (2022) Dispersal patterns of the endangered Crested Ibis suggest high breeding densities drive natal dispersal, Ornithol Appl (published online, https://doi.org/10.1093/ornithapp/duac042)
Zeng J, Qin B, Lu J, Song Z, Ding C (2017) The growth rate of the wild Crested Ibis Nipponia nippon. Chinese J Zool 52:777–782
Acknowledgements
We are grateful to the staffs of Shaanxi Hanzhong Crested Ibis National Nature Reserve for their field assistance. We thank Dr. Canshi Hu and Yongjie Huang for their contribution in data collection and Dr. Xingfeng Si for his constructive comments on this paper. We thank Christina Riehl and one anonymous reviewer for their valuable comments and suggestions which improved the quality of our paper substantially.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 32270554 and 31900371) and the Biodiversity Survey, Monitoring and Assessment Project of Ministry of Ecology and Environment, China (No. 2019HB2096001006).
Author information
Authors and Affiliations
Contributions
CD and XX conceived the study; YY, CW, BQ, and ZS contributed to the data collection; XX and YY analyzed the data; CD and XX led the writing of the manuscript; EB advised on manuscript writing and reviewed the drafts of the paper.
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the use of animals were followed. All fieldwork was conducted with the permission and cooperation of Shaanxi Hanzhong Crested Ibis National Nature Reserve and approved by the Ethic and Animal Welfare Committee of Beijing Forestry University (Approval No. EAWC_BJFU_2022010). This work was part of a long-term, ongoing conservation and research project for endangered Crested Ibis, which does not have any harmful influence to the Crested Ibis.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by J. Mann
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, X., Ye, Y., Briggs, E. et al. Why do parents produce small broods of offspring that have lower body mass, survival, and lifetime reproductive success? A case study in a long-lived bird. Behav Ecol Sociobiol 77, 30 (2023). https://doi.org/10.1007/s00265-023-03301-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03301-1