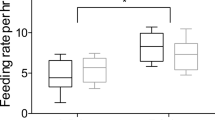
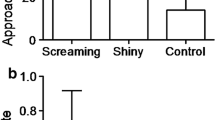
Abstract
Obligate brood parasites rely upon unrelated hosts to provide parental care for their offspring. Specialist brood parasites use visual and vocal mimicry of host offspring to induce host parents to care for the parasite offspring. Generalist brood parasites, however, use different tactics to obtain host parental care. Here, we examine how brown-headed cowbirds (Molothrus ater) restructure host red-winged blackbird (Agelaius phoeniceus) broods and the consequent effects on the expected survival of the brood parasite nestling. Competition within the host brood is governed by an asymmetric sibling rivalry, with the size of the core brood driving the survival of both core and marginal nestlings. Competitively inferior marginal nestlings conversely have little effect on core nestlings. Cowbirds primarily removed core host nestlings when altering the size and structure of host broods. Host brood size at hatching was reduced in conjunction with parasitism by cowbirds. The resulting brood structures differed markedly from those in unparasitized broods with most notably fewer core nestlings, the most potent competitors for the cowbird nestling. Marginal brood size, however, differed only slightly. This reflects the competitive dynamics: core but not marginal brood size affected cowbird survival. The same asymmetric nestling rivalry that governs competition among host nestlings applies to these brood parasitic cowbirds as well; by altering host family structure, they enhanced the effective environment for their nestlings.
Significance statement
Obligate brood parasites must purloin parental care from unrelated host species to rear offspring. Generalist brood parasites, such as cowbirds, face an especially steep challenge, as the offspring are not visual or vocal mimics of host offspring: they are inserted into the host brood and must compete against host offspring. Here, we show that changes in host brood size and structure associated with cowbird parasitism enhance the effective environment for the brood parasite nestling. Through the removal/damage of host eggs or interference with host egg hatching, host brood size is reduced, and most importantly, the number of first-hatched “core” offspring that are the most potent competitors for the brood parasite offspring. The same asymmetric sibling rivalry that exists within the host brood extends to competition between host nestlings and brood parasites.


Similar content being viewed by others
Data availability
The data for the paper are available at: https://dataverse.scholarsportal.info/privateurl.xhtml?token=90060b78-200c-4d3a-a85d-d781d0325115 (brood structure); https://dataverse.scholarsportal.info/privateurl.xhtml?token=daac8b1a-29b6-48b5-a5a1-09854a030fc8 (cowbird nestling survival); https://dataverse.scholarsportal.info/privateurl.xhtml?token=4b027565-f750-4e26-9523-eaa02300288b (egg removal analysis); https://dataverse.scholarsportal.info/privateurl.xhtml?token=f3a49627-c4a7-465c-9a81-c84c6cbc3b06 (demographic data).
References
Briskie JV, Naugler CT, Leech SM (1994) Begging intensity of nestling birds varies with sibling relatedness. Proc R Soc Lond B 258:73–78
Briskie JV, Sealy SG (1990) Evolution of short incubation periods in the parasitic cowbirds, Molothrus spp. Auk 107:789–794
Brooke MDL, Davies NB (1988) Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335:630–632
Clark AB, Wilson DS (1981) Avian breeding adaptations: hatching asynchrony, brood reduction, and nest failure. Q Rev Biol 56:253–277
Davies NB (2000) Cuckoos, cowbirds and other cheats. T & AD Poyser, London
Dearborn DC (1998) Begging behavior and food acquisition by brown-headed cowbird nestlings. Behav Ecol Sociobiol 43:259–270
Fiorini VD, Gloag R, Kacelnik A, Reboreda JC (2014) Strategic egg destruction by brood-parasitic cowbirds? Anim Behav 93:229–235
Fiorini VD, Reboreda JC (2006) Cues used by shiny cowbirds (Molothrus bonariensis) to locate and parasitise chalk-browed mockingbird (Mimus saturninus) nests. Behav Ecol Sociobiol 60:379–385
Fiorini VD, Tuero DT, Reboreda JC (2009) Shiny cowbirds synchronize parasitism with host laying and puncture host eggs according to host characteristics. Anim Behav 77:561–568
Forbes S (2009) Portfolio theory and how parent birds manage investment risk. Oikos 118:1561–1569
Forbes S (2010) Family structure and variation in reproductive success in blackbirds. Behav Ecol Sociobiol 64:475–483
Forbes S (2011) Social rank governs the effective environment of siblings. Biol Lett 7:346–348
Forbes S (2013) Why offspring in nonhuman families differ. Evol Psychol 11:493–512
Forbes S, Glassey B (2000) Asymmetric sibling rivalry and nestling growth in red-winged blackbirds (Agelaius phoeniceus). Behav Ecol Sociobiol 48:413–417
Forbes S, Thornton S, Glassey B, Forbes M, Buckley NJ (1997) Why parent birds play favourites. Nature 390:351–352
Glassey B, Forbes S (2003) Why brown-headed cowbirds do not influence red-winged blackbird parent behaviour. Anim Behav 65:1235–1246
Gloag R, Tuero DT, Fiorini VD, Reboreda JC, Kacelnik A (2011) The economics of nestmate killing in avian brood parasites: a provisions trade-off. Behav Ecol 23:132–140
Hauber ME (2003a) Lower begging responsiveness of host versus parasitic Brown-headed Cowbird (Molothrus ater) nestlings is related to species identity but not to early social experience. J Comp Psychol 117:24–30
Hauber ME (2003b) Hatching asynchrony, nestling competition, and the cost of interspecific brood parasitism. Behav Ecol 14:227–235
Hoover JP, Robinson SK (2007) Retaliatory mafia behavior by a parasitic cowbird favors host acceptance of parasitic eggs. Proc Natl Acad Sci USA 104:4479–4483
Kattan GH (1996) Growth and provisioning of shiny cowbird and house wren host nestlings. J Field Ornithol 67:434–441
Kilner RM (2005) The evolution of virulence in brood parasites. Ornithol Sci 4:55–64
Kilner RM, Madden JR, Hauber ME (2004) Brood parasitic cowbird nestlings use host young to procure resources. Science 305:877–879
Kilner RM, Noble DG, Davies NB (1999) Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature 397:667–672
Lack D (1947) The significance of clutch-size. Ibis 89:302–352
Lack D (1954) The natural regulation of animal numbers. Clarendon Press, Oxford
Langmore NE, Maurer G, Adcock GJ, Kilner RM (2008) Socially acquired host-specific mimicry and the evolution of host races in Horsfield’s bronze-cuckoo Chalcites basalis. Evolution 62:1689–1699
López AV, Fiorini VD, Ellison K, Peer BD (2018) Thick eggshells of brood parasitic cowbirds protect their eggs and damage host eggs during laying. Behav Ecol 29:965–973
Lowther PE (1993) Brown-headed cowbird (Molothrus ater). The birds of North America No. 47. The Academy of Natural Sciences, Philadelphia
Magrath RD (1990) Hatching asynchrony in altricial birds. Biol Rev 65:587–622
Massoni V, Reboreda JC (1999) Egg puncture allows shiny cowbirds to assess host egg development and suitability for parasitism. Proc R Soc Lond B 266:1871–1874
McMaster DG, Sealy SG (1997) Host-egg removal by brown-headed cowbird: a test of the host incubation limit hypothesis. Auk 114:212–220
Payne RB (1977) The ecology of brood parasitism. Annu Rev Ecol Syst 8:1–28
Peer BD (2006) Egg destruction and egg removal by avian brood parasites: adaptiveness and consequences. Auk 123:16–22
Rivers JW (2006) Nest mate size, but not short-term need, influences begging behavior of a generalist brood parasite. Behav Ecol 18:222–230
Rivers JW, Blundell MA, Loughin TM, Peer BD, Rothstein SI (2013) The exaggerated begging behaviour of an obligate avian brood parasite is shared with a nonparasitic close relative. Anim Behav 86:529–536
Rothstein SI, Robinson SK (1998) Parasitic birds and their hosts: studies in coevolution. Oxford University Press, New York
Sealy SG (1992) Removal of Yellow Warbler eggs in association with cowbird parasitism. Condor 94:40–54
Strausberger BM (1998) Temperature, egg mass, and incubation time: a comparison of brown-headed cowbirds and red-winged blackbirds. Auk 115:843–850
Tanaka KD, Ueda K (2005) Horsfield’s hawk-cuckoo nestlings simulate multiple gapes for begging. Science 308:653
Weatherhead PJ, Dufour KW (2000) Fledging success as an index of recruitment in red-winged blackbirds. Auk 117:627–633
Wiebe M (2017) Adaptations and constraints in a generalist obligate brood parasite. MSc Thesis, Dept. of Biology, University of Winnipeg, Winnipeg, Manitoba
Acknowledgements
The authors thank a very large number of field assistants—too numerous to name here—who made this study possible. We thank Douglas Mock and two anonymous reviewers for providing constructive comments that significantly improved the manuscript.
Funding
Funding for this work was provided by the Natural Sciences and Engineering Research Council of Canada and the University of Winnipeg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The work reported here complied with the laws of Canada, and ethical approval was obtained under Animal Care Protocols issued by the University of Winnipeg. All applicable international, national, and/or institutional guidelines for the use of animals were followed.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by M. Soler.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Forbes, S., Glassey, B. & Wiebe, M. Asymmetric sibling rivalry extends to hosts and brood parasites. Behav Ecol Sociobiol 76, 43 (2022). https://doi.org/10.1007/s00265-022-03137-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03137-1