Abstract
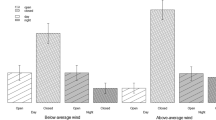
In heterogenous environments, predation risk from multiple predators and the availability of resources fluctuate both spatially and temporally. The various predators may include both aerial and terrestrial species that can facilitate each other and present qualitatively different risks to prey. Animals therefore forage across a complex landscape of fear, with areas of risks and relative safety where resources are generally asymmetrically distributed. Therefore, a trade-off exists between remaining safe and locating food. Animals make foraging decisions regarding where, when and for how long to forage by titrating marginal costs and benefits of foraging within and the marginal value of foraging across depletable resource patches. We conducted a series of titration experiments to determine how Allenby’s gerbils (Gerbillus andersoni allenbyi) titrated food and safety when presented with predation risk from owls, vipers and the joint risk from both predators. We manipulated bush and open microhabitats by increasing food availability in the riskier patches. In response to the different levels of enrichment, gerbils titrated food and safety. Riskier open microhabitats needed to be four times as rich in food as bush patches to be of equal value when subjected to predation from owls and the joint risk from owls and vipers. In response to vipers alone, riskier bush patches needed to be 2–4 times as rich in food as safer open patches for the marginal value of foraging to equalize across microhabitats. Overall, predation risk from owls and the joint risk from owls and vipers resulted in the greatest foraging costs for gerbils in risky microhabitats. Thus, the combined overall risk from multiple predator species was equivalent to the risk presented by the gerbils’ most dangerous predator (owls alone).
Significance statement
Animals trade-off remaining safe with locating food by titrating the marginal costs and benefits of foraging. We assessed how foragers titrate these costs and benefits through behavioural titration experiments. In our study, gerbils titrated food and safety in response to owls and vipers, aerial and terrestrial predators known to facilitate one another. Riskier patches needed to be 2–4 times as rich in food as safer patches to be of equal value to foraging gerbils. From the gerbils’ perspective, owls are more formidable predators than vipers; despite the facilitation that occurs between these predators, the combination of the two predators had little effect on the titration beyond that of the owls alone. Behavioural titrations are important for quantifying differences in foraging costs between microhabitats and in response to multiple predators with different hunting strategies. As single-predator systems are relatively rare, titration experiments provide the opportunity to gain insights into the foraging/safety trade-offs made by animals in response to multiple predators on the landscape.


Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abramsky Z, Strauss E, Subach A, Riechman A, Kotler B (1996) The effect of barn owls (Tyto alba) on the activity and microhabitat selection of Gerbillus allenbyi and G. pyramidum. Oecologia 105:313–319
Abramsky Z, Rosenweig ML, Subach A (1998) Do gerbils care more about competition or predation? Oikos 83:75–84
Abramsky Z, Rosenzweig ML, Subach A (2002) The costs of apprehensive foraging. Ecology 83:1330–1340
Bates D, Maechler M, Bolker B (2012) lme4: Linear mixed-effects models using S4 classes (2011). R package version 0.999375–42. http://cran.r-project.org/web/packages/lme4/index.html
Berger-Tal O, Kotler BP (2010) State of emergency: behavior of gerbils is affected by the hunger state of their predators. Ecology 91:593–600
Berger-Tal O, Mukherjee S, Kotler BP, Brown JS (2010) Complex state-dependent games between owls and gerbils. Ecol Lett 13:302–310
Berger-Tal O, Embar K, Kotler BP, Saltz D (2015) Everybody loses: intraspecific competition induces tragedy of the commons in Allenby’s gerbils. Ecology 96:54–61
Bleicher SS, Brown JS, Embar K, Kotler BP (2016) Novel predator recognition by Allenby’s gerbil (Gerbillus andersoni allenbyi): do gerbils learn to respond to a snake that can “see” in the dark? Isr J Ecol Evol 62:178–185
Bleicher SS, Kotler BP, Shalev O, Dixon A, Embar K, Brown JS (2018) Divergent behavior amid convergent evolution: a case of four desert rodents learning to respond to known and novel vipers. PLoS One 13:e0200672
Blumstein DT, Daniel JC, Springett BP (2004) A test of the multi-predator hypothesis: rapid loss of antipredator behavior after 130 years of isolation. Ethology 110:919–934
Brown JS (1988) Patch use as an indicator of habitat preference, predation risk, and competition. Behav Ecol Sociobiol 22:37–47
Brown JS (1999) Vigilance, patch use and habitat selection: foraging under predation risk. Evol Ecol Res 1:49–71
Brown JS, Kotler BP (2004) Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014
Brown JS, Mitchell WA (1989) Diet selection on depletable resources. Oikos 54:33–43
Brown JS, Kotler BP, Mitchell WA (1994) Foraging theory, patch use, and the structure of a Negev Desert granivore community. Ecology 75:2286–2300
Chamaillé-Jammes S, Malcuit H, Le Saout S, Martin J-L (2014) Innate threat-sensitive foraging: black-tailed deer remain more fearful of wolf than of the less dangerous black bear even after 100 years of wolf absence. Oecologia 174:1151–1158
Creel S, Winnie J, Maxwell B, Hamlin K, Creel M (2005) Elk alter habitat selection as an antipredator response to wolves. Ecology 86:3387–3397
Cresswell W, Lind J, Quinn JL (2010) Predator-hunting success and prey vulnerability: quantifying the spatial scale over which lethal and non-lethal effects of predation occur. J Anim Ecol 79:556–562
Embar K, Kotler BP, Mukherjee S (2011) Risk management in optimal foragers: the effect of sightlines and predator type on patch use, time allocation, and vigilance in gerbils. Oikos 120:1657–1666
Embar K, Raveh A, Hoffmann I, Kotler BP (2014) Predator facilitation or interference: a game of vipers and owls. Oecologia 174:1301–1309
Emerson SE, Kotler BP, Sargunaraj F (2018) Foraging efficiency in the face of predation risk: a comparative study of desert rodents. Evol Ecol Res 19:61–70
Enstam KL, Isbell LA (2004) Microhabitat preference and vertical use of space by patas monkeys (Erythrocebus patas) in relation to predation risk and habitat structure. Folia Primatol 75:70–84
Fretwell SD, Lucas HL (1969) On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor 19:16–36
Holbrook SJ, Schmitt RJ (1988) The combined effects of predation risk and food reward on patch selection. Ecology 69:125–134
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Juliana JRS, Kotler BP, Brown JS, Mukherjee S, Bouskila A (2011) The foraging response of gerbils to a gradient of owl numbers. Evol Ecol Res 13:869–878
Kotler BP (1992) Behavioral resource depression and decaying perceived risk of predation in two species of coexisting gerbils. Behav Ecol Sociobiol 30:239–244
Kotler BP, Blaustein L (1995) Titrating food and safety in a heterogeneous environment: when are the risky and safe patches of equal value? Oikos 74:251–258
Kotler BP, Brown JS (1990) Rates of seed harvest by two species of gerbilline rodents. J Mammal 71:591–596
Kotler BP, Brown JS, Smith RJ, Wirtz WO (1988) The effects of morphology and body size on rates of owl predation on desert rodents. Oikos 53:145–152
Kotler BP, Brown JS, Hasson O (1991) Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72:2249–2260
Kotler BP, Blaustein L, Brown JS (1992) Predator facilitation: the combined effect of snakes and owls on the foraging behavior of gerbils. Ann Zool Fenn 29:199–206
Kotler BP, Brown JS, Slotow RH, Goodfriend WL, Strauss M (1993a) The influence of snakes on the foraging behavior of gerbils. Oikos 67:309–316
Kotler BP, Brown JS, Subach A (1993b) Mechanisms of species coexistence of optimal foragers: temporal partitioning by two species of sand dune gerbils. Oikos 67:548–556
Kotler BP, Brown JS, Oldfield A, Thorson J, Cohen D (2001) Foraging substrate and escape substrate: patch use by three species of gerbils. Ecology 82:1781–1790
Kotler BP, Brown JS, Bouskila A (2004) Apprehension and time allocation in gerbils: the effects of predatory risk and energetic state. Ecology 85:917–922
Kotler BP, Brown J, Mukherjee S, Berger-Tal O, Bouskila A (2010) Moonlight avoidance in gerbils reveals a sophisticated interplay among time allocation, vigilance and state-dependent foraging. Proc R Soc Lond B 277:1469–1474
Laundré JW, Hernández L, Altendorf KB (2001) Wolves, elk, and bison: reestablishing the"landscape of fear" in Yellowstone National Park, USA. Can J Zool 79:1401–1409
Laundré JW, Hernández L, Ripple WJ (2010) The landscape of fear: ecological implications of being afraid. Open Ecol J 3:1–7
Lima SL (2002) Putting predators back into behavioral predator–prey interactions. Trends Ecol Evol 17:70–75
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lindshield S, Danielson BJ, Rothman JM, Pruetz JD (2017) Feeding in fear? How adult male western chimpanzees (Pan troglodytes verus) adjust to predation and savanna habitat pressures. Am J Phys Anthropol 163:480–496
Lone K, Loe LE, Gobakken T, Linnell JD, Odden J, Remmen J, Mysterud A (2014) Living and dying in a multi-predator landscape of fear: roe deer are squeezed by contrasting pattern of predation risk imposed by lynx and humans. Oikos 123:641–651
Longland WS, Price MV (1991) Direct observations of owls and heteromyid rodents: can predation risk explain microhabitat use? Ecology 72:2261–2273
Makin DF, Chamaillé-Jammes S, Shrader AM (2017) Changes in feeding behavior and patch use by herbivores in response to the introduction of a new predator. J Mammal 99:341–350
McIntosh AR, Peckarsky BL (1999) Criteria determining behavioural responses to multiple predators by a stream mayfly. Oikos 85:554–564
Mitchell WA, Abramsky Z, Kotler BP, Pinshow B, Brown JS (1990) The effect of competition on foraging activity in desert rodents: theory and experiments. Ecology 71:844–854
Morosinotto C, Villers A, Varjonen R, Korpimäki E (2017) Food supplementation and predation risk in harsh climate: interactive effects on abundance and body condition of tit species. Oikos 126:863–873
Nicholson KL, Milleret C, Månsson J, Sand H (2014) Testing the risk of predation hypothesis: the influence of recolonizing wolves on habitat use by moose. Oecologia 176:69–80
Ovadia O, zu Dohna H (2003) The effect of intra-and interspecific aggression on patch residence time in Negev Desert gerbils: a competing risk analysis. Behav Ecol 14:583–591
Padié S, Morellet N, Hewison A, Martin JL, Bonnot N, Cargnelutti B, Chamaillé-Jammes S (2015) Roe deer at risk: teasing apart habitat selection and landscape constraints in risk exposure at multiple scales. Oikos 124:1536–1546
Pettersson LB, Brönmark C (1993) Trading off safety against food: state dependent habitat choice and foraging in crucian carp. Oecologia 95:353–357
Preisser EL, Orrock JL, Schmitz OJ (2007) Predator hunting mode and habitat domain alter nonconsumptive effects in predator–prey interactions. Ecology 88:2744–2751
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355
Sinclair AR (1985) Does interspecific competition or predation shape the African ungulate community? J Anim Ecol:899–918
Stears K, Shrader AM (2015) Increases in food availability can tempt oribi antelope into taking greater risks at both large and small spatial scales. Anim Behav 108:155–164
Thaker M, Vanak AT, Owen CR, Ogden MB, Niemann SM, Slotow R (2011) Minimizing predation risk in a landscape of multiple predators: effects on the spatial distribution of African ungulates. Ecology 92:398–407
Tolon V, Dray S, Loison A, Zeileis A, Fischer C, Baubet E (2009) Responding to spatial and temporal variations in predation risk: space use of a game species in a changing landscape of fear. Can J Zool 87:1129–1137
Venables WN, Ripley BD (2002) Random and mixed effects. In: Modern applied statistics with S. Springer, Berlin, pp 271–300
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects modelling for nested data. In: Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (eds) Mixed effects models and extensions in ecology with R. Springer, Berlin, pp 101–142
Acknowledgements
We would like to thank Stuart Summerfield for developing our RFID system. Six anonymous reviewers made constructive comments on the manuscript. DFM received financial support as a recipient of a postdoctoral fellowship from the Jacob Blaustein Center for Scientific Cooperation. This is publication 1052 from the Mitrani Department of Desert Ecology.
Funding
This work was supported by the Israel Science Foundation (grant number: 976/14).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All experiments conducted were compliant with the Ben-Gurion University of the Negev Animal Ethics Committee (IL-09-02-19(D)), IACUC guidelines and the Israel Nature and Parks Authority. Gerbils were released into the vivarium 4 days prior to the start of the experiment to eliminate the impacts of any human-induced stress caused during their transfer. Thus, gerbil behaviour was considered normal before any predators were allowed access to the vivarium.
Additional information
Communicated by T. Stankowich
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Makin, D.F., Kotler, B.P. How do Allenby’s gerbils titrate risk and reward in response to different predators?. Behav Ecol Sociobiol 74, 6 (2020). https://doi.org/10.1007/s00265-019-2785-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2785-6