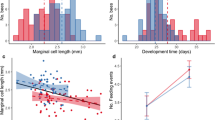
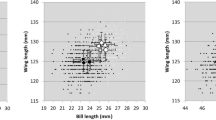
Abstract
Although biased offspring sex ratios are common in species with sexual size dimorphism, the proximate causes are often unresolved. This is because two general mechanisms operating in different ways and in various periods of reproduction can lead to the bias: sex-biased survival or parental sex-allocation. We examined nestling sex ratio patterns between hatching and fledging, sexual size dimorphism, and factors affecting nestling survival using growth and survival data of 846 individual little owl Athene noctua nestlings with known sex from 307 broods from Germany, the Netherlands and Denmark. Nestling sex ratio was female-biased, mainly due to a significant female bias in the first-hatched chicks. Females showed a higher body weight than male nestlings at ringing and body weight of nestlings decreased with hatching sequence. Nestling survival was higher in females (Φ = 0.91) than in males (Φ = 0.85), and survival rates were positively related to body mass and negatively to brood size. Although the observed lower survival of males can cause an overall female-biased sex ratio, the sex dimorphism and survival patterns found here are unlikely to explain the conspicuous sex ratio pattern with a female bias in the first-hatched nestlings and the increase in female bias across the season. Thus, these results point towards interacting mechanisms of parental sex allocation strategies and sex-specific survival. As the female bias was allocated to the first rank that is most likely to survive, the female bias will increase under suboptimal breeding conditions. We therefore suggest that under suboptimal ecological conditions, higher investment into females is adaptive in little owls.
Significance statement
Biased sex ratios can have severe effects on the social behaviour and population dynamics of endangered species. However, the existence of subtle sex ratio bias is often unknown and its proximate mechanisms and ultimate consequences often remain unclear. Small sample sizes make the detection of subtle effects unlikely and often fail to disentangle diverging mechanisms such as sex-biased survival and parental sex allocation. We used a large dataset of 846 little owl nestlings from 307 broods from three countries to investigate offspring sex ratio patterns, sexual size dimorphism and nestling survival simultaneously. Our findings suggest interacting mechanisms of parental sex allocation strategies and sex-specific survival to drive biased offspring sex ratios in little owls. The context dependence of the sex ratio bias indicates that offspring sex ratio bias in little owls is both, a consequence of—and an adaptation to—suboptimal breeding conditions.




Similar content being viewed by others
References
Anderson DJ, Budde C, Apanius V, Gomez JEM, Bird DM, Weathers WW (1993) Prey size influences female competitive dominance in nestling American kestrels (Falco sparverius). Ecology 74:367–376
Apolloni N, Grüebler MU, Arlettaz R, Gottschalk TK, Naef-Daenzer B (2018) Habitat selection and range use of little owls in relation to habitat patterns at three spatial scales. Anim Conserv 2:65–75
Arroyo B (2002) Sex-biased nestling mortality in the Montagu’s harrier Circus pygargus. J Avian Biol 33:455–460
Badyaev AV (2002) Sex-biased hatching order and adaptive population divergence in a passerine bird. Science 295:316–318
Badyaev AV, Oh KP, Mui R (2006) Evolution of sex-biased maternal effects in birds: II. Contrasting sex-specific oocyte clustering in native and recently established populations. J Evol Biol 19:909–921
Bates D, Maechler M, Bolker BM, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bednarz JC, Hayden TJ (1991) Skewed brood sex ratio and sex-biased hatching sequence in Harris’s hawks. Am Nat 137:116–132
Blanco G, Martínez-Padilla J, Dávila JA, Serrano D, Viñuela J (2003) First evidence of sex differences in the duration of avian embryonic period: consequences for sibling competition in sexually dimorphic birds. Behav Ecol 14:702–706
Bollinger PB (1994) Relative effects of hatching order, egg-size variation, and parental quality on chick survival in common terns. Auk 111:263–273
Bortolotti GR (1986) Influence of sibling competition on nestling sex ratios of sexually dimorphic birds. Am Nat 127:495–507
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Carranza J (2004) Sex allocation within broods: the intrabrood sharing-out hypothesis. Behav Ecol 15:223–232
Cichoń M, Sendecka J, Gustafsson L (2005) Male-biased sex ratio among unhatched eggs in great tit Parus major, blue tit P. caeruleus and collared flycatcher Ficedula albicollis. J Avian Biol 36:386–390
Clotfelter ED, Whittingham LA, Dunn PO (2003) Laying order, hatching asynchrony and nestling body mass in tree swallows Tachycineta bicolor. J Avian Biol 31:329–334
Cordero PJ, Viñuela J, Aparicio JM, Veiga JP (2001) Seasonal variation in sex ratio and sexual egg dimorphism favouring daughters in first clutches of the spotless starling. J Evol Biol 14:829–834
Core Team R (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://www.r-project.org/
Daan S, Dijkstra C, Weissing FJ (1996) An evolutionary explanation for seasonal trends in avian sex ratios. Behav Ecol 7:426–430
Darwin C (1871) The descent of man and selection in relation to sex. John Murray, London
Dijkstra C, Daan S, Buker JB (1990) Adaptive seasonal variation in the sex ratio of kestrel broods. Funct Ecol 4:143–147
Donald PF (2007) Adult sex ratios in wild bird populations. Ibis 149:671–692
Droge DL, Gowaty PA, Weathers WW (1991) Sex-biased provisioning: a test for differences in field metabolic rates of nestling eastern bluebirds. Condor 93:793–798
Eberhart-Phillips LJ, Küpper C, Carmona-Isunza MC, Vincze O, Zefania S, Cruz-López M, Kosztolányi A, Miller TEX, Barta Z, Cuthill IC, Burke T, Székely T, Hoffman JI, Krüger O (2018) Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nat Commun 9:1651
Espíndola-Hernández P, Castaño-Villa GJ, Vásquez RA, Quirici V (2017) Sex-specific provisioning of nutritious food items in relation to brood sex ratios in a non-dimorphic bird. Behav Ecol Sociobiol 71:65
Fiala KL, Congdon JD (1983) Energetic consequences of sexual size dimorphism in nestling red-winged blackbirds. Ecology 64:642–647
Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford
Fletcher KL, Hamer KC (2004) Offspring sex ratio in the common tern Sterna hirundo, a species with negligible sexual size dimorphism. Ibis 146:454–460
Gelman A, Hill J (2007) Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press, Cambridge.
Gilby AJ, Sorato E, Griffith SC (2012) Maternal effects on begging behaviour: an experimental demonstration of the effects of laying sequence, hatch order, nestling sex and brood size. Behav Ecol Sociobiol 66:1519–1529
Grüebler MU, Müller M, Michel VT, Perrig M, Keil H, Naef-Daenzer B, Korner-Nievergelt F (2018) Brood provisioning and reproductive benefits in relation to habitat quality: a food supplementation experiment. Anim Behav 141:45–55
Hardy ICW (1997) Possible factors influencing vertebrate sex ratios: an introductory overview. Appl Anim Behav Sci 51:217–241
Hasselquist D, Kempenaers B (2002) Parental care and adaptive brood sex ratio manipulation in birds. Phil Trans R Soc B 357:363–372
Hipkiss T, Hörnfeldt B, Eklund U, Berlin S (2002) Year-dependent sex-biased mortality in supplementary-fed Tengmalm’s owl nestlings. J Anim Ecol 71:693–699
Hjernquist MB, Thuman Hjernquist KA, Forsman JT, Gustafsson L (2009) Sex allocation in response to local resource competition over breeding territories. Behav Ecol 20:335–339
Hurst J (2009) Die Populationsgenetik des Steinkauzes (Athene noctua) in Süddeutschland und angrenzenden Gebieten. Diploma thesis. Albert-Ludwigs-Universität, Freiburg
Juillard M (1979) La croissance des jeunes Chouettes chevêches, Athene noctua, pendant leur séjour au nid. Nos Oiseaux 35:113–124
Julliard R (2000) Sex-specific dispersal in spatially varying environments leads to habitat-dependent evolutionary stable offspring sex ratios. Behav Ecol 11:421–428
Kilner R (1998) Primary and secondary sex ratio manipulation by zebra finches. Anim Behav 56:155–164
Komdeur J, Pen I (2002) Adaptive sex allocation in birds: the complexities of linking theory and practice. Phil Trans R Soc B 357:373–380
Korner-Nievergelt F, Roth T, von Felten S, Guélat J, Almasi B, Korner-Nievergelt P (2015) Bayesian data analysis in ecology using linear models with R, BUGS, and Stan. Elsevier, New York
Krijgsveld KL, Dijkstra C, Visser GH, Daan S (1998) Energy requirements for growth in relation to sexual size dimorphism in marsh harrier Circus aeruginosus nestlings. Physiol Zool 71:693–702
Leimar O (1996) Life-history analysis of the Trivers and Willard sex-ratio problem. Behav Ecol 7:316–325
Magrath RD (1990) Hatching asynchrony in altricial birds. Biol Rev 65:587–622
Magrath RD (1991) Nestling weight and juvenile survival in the blackbird, Turdus merula. J Anim Ecol 60:335–351
Mead PS, Morton ML, Fish BE (1987) Sexual dimorphism in egg size and implications regarding facultative manipulation of sex in mountain white-crowned sparrows. Condor 89:798–803
Michel (2016) Individual responses of adult little owls (Athene noctua) to environmental conditions. PhD thesis, University of Zurich
Michel VT, Naef-Daenzer B, Keil H, Grüebler MU (2017) Reproductive consequences of farmland heterogeneity in little owls (Athene noctua). Oecologia 183:1019–1029
Michler SPM, Nicolaus M, Ubels R, van der Velde M, Komdeur J, Both C, Tinbergen JM (2011) Sex-specific effects of the local social environment on juvenile post-fledging dispersal in great tits. Behav Ecol Sociobiol 65:1975–1986
Morrison CA, Robinson RA, Clark JA, Gill JA (2016) Causes and consequences of spatial variation in sex ratios in a declining bird species. J Anim Ecol 85:1298–1306
Neto JM, Hansson B, Hasselquist D (2011) Sex allocation in Savi’s warblers Locustella luscinioides: multiple factors affect seasonal trends in brood sex ratios. Behav Ecol Sociobiol 65:297–304
Nicolaus M, Michler SPM, Ubels R, van der Velde M, Komdeur J, Both C, Tinbergen JM (2009) Sex-specific effects of altered competition on nestling growth and survival: an experimental manipulation of brood size and sex ratio. J Anim Ecol 78:414–426
Oddie KR (2000) Size matters: competition between male and female great tit offspring. J Anim Ecol 69:903–912
Penteriani V, Delgado MM, Pérez-García JM et al (2010) Sex allocation from an owl perspective: clutch order could determine brood sex to reduce sibling aggression in the eagle owl Bubo bubo. Ornis Fenn 87:135–143
Perrig M, Grüebler MU, Keil H, Naef-Daenzer B (2014) Experimental food supplementation affects the physical development, behaviour and survival of little owl Athene noctua nestlings. Ibis 156:755–767
Perrig M, Grüebler MU, Keil H, Naef-Daenzer B (2017) Post-fledging survival of little owls Athene noctua in relation to nestling food supply. Ibis 159:532–540
Rutkowska J, Badyaev AV (2008) Meiotic drive and sex determination: molecular and cytological mechanisms of sex ratio adjustment in birds. Phil Trans R Soc B 363:1675–1686
Teather KL, Weatherhead PJ (1994) Allometry, adaptation, and the growth and development of sexually dimorphic birds. Oikos 71:515–525
Thorup K, Sunde P, Jacobsen LB, Rahbek C (2010) Breeding season food limitation drives population decline of the little owl Athene noctua in Denmark. Ibis 152:803–814
Uller T (2006) Sex-specific sibling interactions and offspring fitness in vertebrates: patterns and implications for maternal sex ratios. Biol Rev 81:207–217
van Harxen R, Stroeken P, Sterringa G (2018) Nieuwe gegevens over de eileg, broeden, uitkomst van de eieren en uitvliegen van de jongen bij de steenuil (Athene noctua). Uilen 8:76–89
Van Nieuwenhuyse D, Génot J-C, Johnson DH (2008) The little owl: conservation, ecology and behavior of Athene noctua. Cambridge University Press, Cambridge
Weatherhead PJ, Teather KL (1991) Are skewed fledgling sex ratios in sexually dimorphic birds adaptive? Am Nat 138:1159–1172
West SA (2002) Constraints in the evolution of sex ratio adjustment. Science 295:1685–1688
Zuur AF, Hilbe JM, Ieno EN (2013) A beginner’s guide to GLM and GLMM with R. Highland Statistics Ltd., Newburgh
Acknowledgments
We thank all field assistants and volunteers, in particular Gerhard Bauer, Wolfgang Graef, Josef Helmik, Thomas Henschel, Rudi Holleitner, Petra Kauder, Klaus Lopitz, Alexander Neu, Jens Polzien, Erwin Reichert, Volker Schlie, Dirk Unkelbach and Bruno Vollmar for their help in collecting data. Furthermore, we thank Fränzi Korner-Nievergelt for statistical support and three anonymous referees for their valuable comments.
Funding
This work was supported by the Swiss National Science Foundation (Grant 3100A 132951/1 to BN-D and MUG), the Hirschmann Foundation and the Karl Mayer Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Handling and ringing of little owl nestlings in Germany was carried out under the permit of the regional council of Baden-Württemberg, Germany (licence No. 35e9185.81/0288), the regional council of Rheinland Pfalz (licence Az 42/553-253) and Stuttgart (licence Az 55-8853.17), the Struktur- und Genehmigungsdirektion (SGD) Süd and SGD Nord of Rheinland-Pfalz, as well as Vogelwarte Radolfzell (licences no. 1146, 1191; 1403 and 1903). Handling and ringing of the nestlings in Denmark was carried out under licence from Copenhagen Bird Ringing Centre (A-392 personal ringing licence to LBJ). The sampling of 5 growing breast feathers in Denmark was permitted by The Animal Experiments Inspectorate (#2011/561-17). Handling and ringing in the Netherlands was carried out under the licence from Vogeltrekstation, Dutch centre for avian migration and demography (R. van Harxen–848). The sampling of growing breast feathers in the Netherlands was permitted by Dierexperimentencommissie Koninklijke Academie van Wetenschappen/NIOO 13. 07 advies. All procedures followed the ASAB/ABS guidelines for the ethical treatment of animals in behavioural research and teaching and all applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The handling of birds was performed with maximum care and disturbance to nests kept to a minimum. Ethical approval for involving animals in this study was received through the application procedure for ringing permits and the scientific commission of the Swiss Ornithological Institute.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by L. Z. Garamszegi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 738 kb)
Rights and permissions
About this article
Cite this article
Tschumi, M., Humbel, J., Erbes, J. et al. Parental sex allocation and sex-specific survival drive offspring sex ratio bias in little owls. Behav Ecol Sociobiol 73, 85 (2019). https://doi.org/10.1007/s00265-019-2694-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2694-8