Abstract
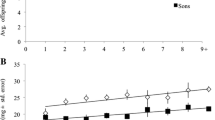
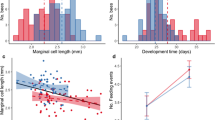
Provisioning for young offspring is an archetypical form of parental investment. Ceratina calcarata bees provide extended maternal care to their young and demonstrate an unusual strategy of dual-phase pollen provisioning. Most bees first gather provisions as they establish nests in spring. However, C. calcarata mothers will also feed their newly eclosed young a second time, perhaps ensuring their survival during a long winter diapause. Some mothers rear a small, worker-like daughter to assist them during this second provisioning phase. We studied provisioning behavior in C. calcarata to examine patterns of maternal investment and foraging dynamics throughout the breeding season. Mothers typically made a high number of short-duration foraging trips each day, whereas late-season females tended to make fewer and longer trips. This difference in foraging duration may indicate a lower risk of brood loss in those nests where mature offspring are present. Nest demographic data revealed that an offspring laid in the first brood cell position is typically female and usually smaller than her siblings. In 29% of the nests, this small daughter was observed to adopt a forager role at maturity and provisioned for her siblings. Dwarf daughters had a higher number of active days and foraging trips per day in orphaned nests than in nests where a mother was present. The foraging behaviors of worker-like daughters were similar in length of foraging trip and handling time to mothers during this second provisioning period. We hypothesize that incipiently social foraging by this smallest daughter may act as a form of insurance against brood loss during occasions when a mother is unable to sufficiently provision for her eclosed offspring during the second phase.
Significance statement
Parental investment in the size and sex of offspring is under strong selection for assured fitness returns. For example, many social insect mothers make an initial investment in small offspring to take on risky foraging behavior while they specialize on future reproduction. Solitary and facultatively social species provide an important baseline to understand the evolution of social complexity from natural variation in maternal care and foraging behavior. Here, we characterize the parental investment strategies of a subsocial small carpenter bee and reveal the potential adaptive significance of prolonged maternal care and worker production in this species. Mothers provide an initial investment that is extended by workers providing alloparental care to siblings. Maternal manipulation of dwarf eldest daughters may serve as an insurance mechanism in the event of maternal mortality to assure the survival of siblings.



Similar content being viewed by others
References
Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383
Bosch J, Vicens N (2002) Body size as an estimator of production costs in a solitary bee. Ecol Entomol 27:129–137
Bourke AF (2011) Principles of social evolution. Oxford University Press, Oxford 280pp
Charnov EL (1982) The theory of sex allocation. Princeton University, Princeton
Cowan DP (1981) Parental investment in two solitary wasps Ancistrocerus adiabatus and Euodynerus foraminatus (Eumenidae: hymenoptera). Behav Ecol Sociobiol 9:95–102
Craig R (1983) Subfertility and the evolution of eusociality by kin selection. J Theor Biol 100:379–397
Crozier RH, Pamilo P (1996) Evolution of social insect colonies. Oxford University Press, Oxford
Danforth BN (1990) Provisioning behavior and the estimation of investment ratios in a solitary bee, Calliopsis (Hypomacrotera) persimilis (Cockerell) (hymenoptera: Andrenidae). Behav Ecol Sociobiol 27:159–168
Durant DR, Berens AJ, Toth AL, Rehan SM (2016) Transcriptional profiling of overwintering gene expression in the small carpenter bee, Ceratina calcarata. Apidologie 47:572–582
Field J (2005) The evolution of progressive provisioning. Behav Ecol 16:770–778
Field J, Shreeves G, Sumner S, Casiraghi M (2000) Insurance-based advantage to helpers in a tropical hover wasp. Nature 404:869–871
Fisher RA (1930) The genetical theory of natural selection. Dover Publications, Inc., New York
Frank SA (1987) Individual and population sex allocation patterns. Theor Popul Biol 31:47–74
Gerber HS, Klostermeyer EC (1970) Sex control by bees: a voluntary act of egg fertilization during oviposition. Science 167:82–84
Hogendoorn K, Velthuis HHW (1999) Task allocation and reproductive skew in social mass provisioning carpenter bees in relation to age and size. Insect Soc 46:198–207
Johnson M (1988) The relationship of provision weight to adult weight and sex-ratio in the solitary bee, Ceratina calcarata. Ecol Entomol 13:165–170
Kapheim KM, Bernal SP, Smith AR, Nonacs P, Wcislo WT (2011) Support for maternal manipulation of developmental nutrition in a facultatively eusocial bee, Megalopta genalis (Halictidae). Behav Ecol Sociobiol 65:1179–1190
Lawson SP, Ciaccio KN, Rehan SM (2016) Maternal manipulation of pollen provisions affects worker production in a small carpenter bee. Behav Ecol Sociobiol 70:1891–1900
Lewis V, Richards MH (2017) Experimentally induced alloparental care in a solitary carpenter bee. Anim Behav 123:229–238
Maeta Y, Sakagami SF (1995) Oophagy and egg replacement in artificially induced colonies of a basically solitary bee, Ceratina (Ceratinidia) okinawana (hymenoptera, Anthophoridae, Xylocopinae), with a comparison of social behavior among Ceratina, Xylocopa and the halictine bees. Jpn J Entomol 63:347–375
Maeta Y, Sugiura N, Goubara M (1992) Patterns of offspring production and sex allocation in the small carpenter bee, Ceratina flavipes smith (hymenoptera, Xylocopinae). Jpn J Ent 60:175–190
Mas F, Kölliker M (2008) Maternal care and offspring begging in social insects: chemical signalling, hormonal regulation and evolution. Anim Behav 76:1121–1131
McFrederick QS, Rehan SM (2016) Characterization of pollen and bacterial community composition in brood provisions of a small carpenter bee. Mol Ecol 25:2302–2311
Michener CD (2007) The bees of the world, 2nd edn. The Johns Hopkins University Press, Baltimore
Michener CD (1974) The social behavior of the bees: a comparative study. Harvard University Press, Cambridge
Mikát M, Černá K, Straka J (2016) Major benefits of guarding behavior in subsocial bees: implications for social evolution. Ecol Evol 6:6784–6797
Molumby A (1997) Why make daughters larger? Maternal sex-allocation and sex-dependent selection for body size in a mass-provisioning wasp, Trypoxylon politum. Behav Ecol 8:279–287
Morato EF, Martins RP (2006) An overview of proximate factors affecting the nesting behavior of solitary wasps and bees (Hymenoptera: Aculeata) in preexisting cavities in wood. Neotrop Entomol 35:285–298
O’Donnell S (1998) Reproductive caste determination in eusocial wasps (Hymenoptera: Vespidae). Annu Rev Entomol 43:323–346
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rehan SM, Richards MH (2010a) The influence of maternal quality on brood sex allocation in the small carpenter bee, Ceratina calcarata. Ethology 116:876–887
Rehan SM, Richards MH (2010b) Nesting biology and subsociality in Ceratina calcarata (Hymenoptera: Apidae). Can Entomol 142:65–74
Rehan SM, Sheffield CS (2011) Morphological and molecular delineation of a new species in the Ceratina dupla species-group (Hymenoptera: Apidae: Xylocopinae) of eastern North America. Zootaxa 2873:35–50
Rehan SM, Richards MH (2013) Reproductive aggression and nestmate recognition in a subsocial bee. Anim Behav 85:733–741
Rehan SM, Richards MH, Schwarz MP (2010) Social polymorphism in the Australian small carpenter bee, Ceratina (Neoceratina) australensis. Insect Soc 57:403–412
Rehan SM, Leys R, Schwarz MP (2012) A mid-cretaceous origin of sociality in xylocopine bees with only two origins of true worker castes indicates severe barriers to eusociality. PLoS One 7:e34690
Rehan SM, Leys R, Schwarz MP (2013) First evidence for a massive extinction event affecting bees close to the K-T boundary. PLoS One 8:e76683
Rehan SM, Berens AJ, Toth AL (2014) At the brink of eusociality: transcriptomic correlates of worker behaviour in a small carpenter bee. BMC Evol Biol 14:260
Richards MH, Course C (2015) Ergonomic skew and reproductive queuing based on social and seasonal variation in foraging activity of eastern carpenter bees (Xylocopa virginica). Can J Zool 93:615–625
Richards MH, Packer L (1996) The socioecology of body size variation in the primitively eusocial sweat bee, Halictus ligatus (Hymenoptera: Halictidae). Oikos 77:68–76
Sakagami SF, Maeta Y (1984) Multifemale nests and rudimentary castes in the normally solitary bee Ceratina japonica (Hymenoptera: Xylocopinae). J Kansas Entomol Soc 57:639–656
Sakagami SF, Maeta Y (1977) Some presumably presocial habits of Japanese Ceratina bees, with notes on various social types in Hymenoptera. Insect Soc 24:319–343
Schwarz MP, Richards MH, Danforth BN (2007) Changing paradigms in insect social evolution: insights from halictine and allodapine bees. Annu Rev Entomol 52:127–150
Seidelmann K (2006) Open-cell parasitism shapes maternal investment patterns in the red mason bee Osmia rufa. Behav Ecol 17:839–848
Shell WA, Rehan SM (2016) Recent and rapid diversification of the small carpenter bees in eastern North America. Biol J Linn Soc 117:633–645
Shreeves G, Cant MA, Bolton A, Field J (2003) Insurance–based advantages for subordinate co–foundresses in a temperate paper wasp. Proc R Soc Lond B Biol Sci 270:1617–1622
Smith AR, Kapheim KM, O’Donnell S, Wcislo WT (2009) Social competition but not subfertility leads to a division of labour in the facultatively social sweat bee Megalopta genalis (Hymenoptera: Halictidae). Anim Behav 78:1043–1050
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Strassmann JE, Lee RE, Rojas RR, Baust JG (1984) Caste and sex differences in cold-hardiness in the social wasps, Polistes annularis and P. exclamans (Hymenoptera: Vespidae). Insect Soc 31:291–301
Strohm E, Linsenmair KE (2000) Allocation of parental investment among individual offspring in the European beewolf Philanthus triangulum F. (Hymenoptera: Sphecidae). Biol J Linn Soc 69:173–192
Strohm E, Linsenmair KE (1999) Measurement of parental investment and sex allocation in the European beewolf Philanthus triangulum F. (Hymenoptera: Sphecidae). Behav Ecol Sociobiol 47:76–88
Tallamy DW, Wood TK (1986) Convergence patterns in subsocial insects. Annu Rev Entomol 31:369–390
Tomkins JL, Simmons LW, Alcock J (2001) Brood-provisioning strategies in Dawson’s burrowing bee, Amegilla dawsoni (Hymenoptera: Anthophorini). Behav Ecol Sociobiol 50:81–89
Toth AL, Bilof KBJ, Henshaw MT, Hunt JH, Robinson GE (2009) Lipid stores, ovary development, and brain gene expression in Polistes metricus females. Insect Soc 56:77–84
Trivers RL, Hare H (1976) Haploidploidy and the evolution of the social insect. Science 191:249–263
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
Vickruck J (2010) The nesting biology of Ceratina (Hymenoptera: Apidae) in the Niagara region: new species, nest site selection and parasitism. Master's Thesis, Department of Biological Sciences, Brock University, St. Catharines
Vickruck JL, Rehan SM, Sheffield CS, Richards MH (2011) Nesting biology and DNA barcode analysis of Ceratina dupla and C. mikmaqi, and comparisons with C. calcarata (Hymenoptera: Apidae: Xylocopinae). Can Entomol 143:254–262
Weissel N, Mitesser O, Poethke HJ, Strohm E (2012) Availability and depletion of fat reserves in halictid foundress queens with a focus on solitary nest founding. Insect Soc 59:67–74
West-Eberhard MJ (1975) The evolution of social behavior by kin selection. Q Rev Biol 50:1–33
Willmer PG, Stone GN (2004) Behavioral, ecological, and physiological determinants of the activity patterns of bees. Adv Study Behav 34:347–466
Wilson EO (1971) The insect societies. Belknap Press of Harvard University Press, Cambridge
Withee JR, Rehan SM (2016) Cumulative effects of body size and social experience on aggressive behaviour in a subsocial bee. Behaviour 153:1365–1385
Acknowledgements
We thank Wyatt Shell and member of the Rehan lab for comments and suggestions on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by a mobility fund of Charles University of Prague and Integrative Animal Biology award no. SVV 260 434/2017 to MM. Additionally, this research was supported by NSF award no. 1456296 to SMR.
Additional information
Communicated by J. Field
Rights and permissions
About this article
Cite this article
Mikát, M., Franchino, C. & Rehan, S.M. Sociodemographic variation in foraging behavior and the adaptive significance of worker production in the facultatively social small carpenter bee, Ceratina calcarata . Behav Ecol Sociobiol 71, 135 (2017). https://doi.org/10.1007/s00265-017-2365-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2365-6