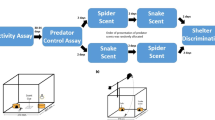
Abstract
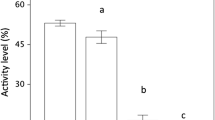
Alien predators may impose a great threat to naïve prey. Ibiza wall lizards (Podarcis pityusensis) live in Ibiza, a snake-free island until 2003. We studied the lizards’ discrimination of scents of two invader snakes: one that depredates lizards, the horseshoe whip snake (Hemorrhois hippocrepis), and another that does not, the ladder snake (Rhinechis scalaris). We compared two populations of Ibiza wall lizards: one from the main island of Ibiza, which coexists with both snakes, and another from the nearby snake-free islet of Sal Rossa. Lizards from Ibiza recognized the scent of the horseshoe whip snake and responded with clear antipredatory behaviours. However, they reacted to the scent of the ladder snake similarly to that of the controls (odourless control and pungent scent). Lizards from Sal Rossa did not respond to any of the snakes or the controls. Our results show that lizards can rapidly acquire the ability to react to a novel predator. As only about ten generations of lizards have coexisted with snakes, the most plausible explanation to our results is that lizards have learned to associate the scent of the predatory snake with a threat. This is the first study reporting the rapid acquisition of lizards’ antipredatory responses to the chemical cues of novel predators. However, more research is needed in order to identify the mechanisms implicated in the response.
Significance statement
How naïve prey acquire antipredator behaviour is both important for basic scientific research and useful for the conservation of native species subjected to biological invasions. The island of Ibiza (Spain) has been free from snakes until their introduction by humans in 2003. We compared the reaction of lizards from Ibiza (where presently three species of snakes cohabitate) and lizards from Sal Rossa (a nearby snake-free islet) to the scent of two snakes (one that feeds on lizards and other that does not). The results were clear: lizards from Ibiza react to the scent of the predatory snake with antipredatory behaviours, while ignoring the scent of the non-predatory snake. Lizards from the snake-free island of Sal Rossa did not react to any of the snakes. Our study shows that lizards can rapidly acquire the ability to react to a completely new type of predator, most likely by learning.

Similar content being viewed by others
References
Alcover JA (1984) Uber die Nahrung der Ginsterkatze Genetta genetta (Linnaeus, 1758) auf der Inseln Mallorca, Ibiza und Cabrera. Saügetierkd Mitt 31:189–195
Alcover JA (2010) A century of insular vertebrate paleontology research on the Balearic Islands. In: Pérez-Mellado V, Ramon C (eds) Islands and evolution. Institut Menorqui d’Estudis, Mao, pp. 59–83
Álvarez C, Mateo JA, Oliver J, Mayol J (2010) Los ofidios ibéricos de introducción reciente en las Islas Baleares. Bol Asoc Herpetol Esp 21:126–131
Amo L, López P, Martín J (2004) Wall lizards combine chemical and visual cues of ambush snake predators to avoid overestimating risk inside refuges. Anim Behav 670:647–653
Anson JR, Dickman CR (2013) Behavioural responses of native prey to disparate predators: naiveté and predator recognition. Oecologia 171:367–377
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29:1123–1144
Arnold EN (1984) Evolutionary aspects of tail shedding in lizards and their relatives. J Nat Hist 18:127–169
Banks PB, Dickman CR (2007) Alien predation and the effects of multiple levels of prey naiveté. Trends Ecol Evol 22:229–230
Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ (2004) Avian extinction and mammalian introductions on oceanic islands. Science 305:1955–1958
Blumstein DT (2002) Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J Biogeogr 29:685–692
Blumstein DT, Daniel JC (2005) The loss of anti-predator behaviour following isolation on islands. Proc R Soc Lond B 272:1663–1668
Brown GE, Chivers DP, Smith RJF (1997) Differential learning rates of chemical versus visual cues of a northern pike by fathead minnows in a natural habitat. Environ Biol Fish 49:89–96
Brown GE, Ferrari MC, Chivers DP (2011) Learning about danger: chemical alarm cues and threat-sensitive assessment of predation risk by fishes. In: Brown C, Laland K, Krause J (eds) Fish cognition and behavior. Wiley-Blackwell, Oxford, pp. 59–80
Brown GE, Ferrari MC, Elvidge CK, Ramnarine I, Chivers DP (2013) Phenotypically plastic neophobia: a response to variable predation risk. Proc R Soc B 280:20122712
Brown RP, Terrasa B, Pérez-Mellado V, Castro JA, Hoskisson PA, Picornell A, Ramon MM (2008) Bayesian estimation of post-Messinian divergence times in Balearic Island lizards. Mol Phylogenet Evol 48:350–358
Carranza S, Arnold EN, Pleguezuelos JM (2005) Phylogeny, biogeography and evolution of two Mediterranean snakes Malpolon monspessulanus and Hemorrhois hippocrepis (Squamata, Colubridae), using mtDNA sequence. Mol Phylogenet Evol 40:532–546
Chivers DP, Brown GE, Smith RJF (1995) Acquired recognition of chemical stimuli from pike, Esox lucius, by brook sticklebacks, Culaea inconstans (Osteichthyes, Gasterosteidae). Ethology 99:234–242
Cooper WE, Pérez-Mellado V (2012) Historical influence of predation pressure on escape by Podarcis lizards in the Balearic Islands. Biol J Linn Soc 107:254–268
Cooper WE, Hawlena D, Pérez-Mellado V (2009) Interactive effect of starting distance and approach speed on escape behavior challenges theory. Behav Ecol 20:542–546
Cooper WE, Pérez-Mellado V, Vitt LJ, Budzynski B (2003) Cologne as a pungency control in tests of lizard chemical discriminations: effects of concentration, brand, and simultaneous and sequential presentation. J Ethol 21:101–106
Cooper WE, Pyron RA, Garland T (2014) Island tameness: living on islands reduces flight initiation distance. P Roy Soc Lond B Bio 281:20133019. doi:10.1098/rspb.2013.3019
Cox JG, Lima SL (2006) Naiveté and an aquatic–terrestrial dichotomy in the effects of introduced predators. Trends Ecol Evol 21:674–680
Crane AL, Ferrari MCO (2013) Social learning of predation risk: a review and prospectus. In: Clark K (ed) Social learning theory: phylogenetic considerations across animal, plant, and microbial taxa. Nova Science Publisher, New York, pp. 53–82
Dial BE, Weldon PJ, Curtis B (1989) Chemosensory identification of snake predators (Phyllorhynchus decurtatus) by banded geckos (Coleonyx variegatus). J Herpetol 3:224–229
Endler JA (1986) Defense against predators. In: Feder ME, Lauder GV (eds) Predator-prey relationships. Perspectives and approaches from the study of lower vertebrates. The University of Chicago Press, Chicago, pp. 109–134
Ferrari MCO, McCormick MI, Allan BJ, Choi R, Ramasamy RA, Johansen JL, Mitchell MD, Chivers DP (2015) Living in a risky world: the onset and ontogeny of an integrated antipredator phenotype in a coral reef fish. Sci Rep 5:15537
Ferrari MCO, Vrtělová J, Brown GE, Chivers DP (2012) Understanding the role of uncertainty on learning and retention of predator information. Anim Cogn 15:807–813
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Freeman AS, Byers JE (2006) Divergent induced responses to an invasive predator in marine mussel populations. Science 313:831–833
Giraudoux P (2012) pgirmess: data analysis in ecology. R package version 1.5.6., http://CRAN.R-project.org/package=pgirmess
Greene HW (1988) Antipredator mechanisms in reptiles. In: Gans C, Huey RB (eds) Biology of Reptilia, Vol. 16. Ecology B: defense and life history. Alan R. Liss, New York, pp. 1–152
Griffin AS (2004) Social learning about predators: a review and prospectus. Anim Learn Behav 32:131–140
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kelley JL, Magurran AE (2003) Learned predator recognition and antipredator responses in fishes. Fish Fish 4:216–226
Kotsackis T (1981) Le lucertole (Lacertidae, Squamata) del Pliocene, Pleistocene e Olocene delle Baleari. Bol Soc Hist Nat Balears 25:135–150
Kovacs EK, Crowther MS, Webb JK, Dickman CR (2012) Population and behavioural responses of native prey to alien predation. Oecologia 168:947–957
Labra A, Niemeyer HM (2004) Variability in the assessment of snake predation risk by Liolaemus lizards. Ethology 110:649–662
Lima SL, Dill LM (1990) Behavioural decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Losos JB, Schoener TW, Langerhans RB, Spiller DA (2006) Rapid temporal reversal in predator-driven natural selection. Science 314:1111
Mencía A, Ortega Z, Pérez-Mellado V (2016) Chemical discrimination of sympatric snakes by the mountain lizard Iberolacerta galani (Squamata: Lacertidae). Herpetol J 26:151–157
Nunes AL, Orizaola G, Laurila A, Rebelo R (2014) Rapid evolution of constitutive and inducible defenses against an invasive predator. Ecology 95:1520–1530
Pérez-Mellado V, Corti C, Lo Cascio P (1997) Tail autotomy and extinction in Mediterranean lizards. A preliminary study of continental and insular populations. J Zool 243:533–541
Pinya S, Carretero MA (2011) The Balearic herpetofauna: a species update and a review on the evidence. Acta Herpetol 6:59–80
Pleguezuelos JM (1998) Elaphe scalaris (Schinz, 1822). In: Ramos MA, Alba J, Bellés X, Golsálbez J, Guerra A, Macpherson E, Martín F, Serrano J, Templado J (eds) Reptiles, 1st edn, Fauna Ibérica, vol 10. Museo Nacional de Ciencias Naturales, CSIC, Madrid, pp. 390–407
Pleguezuelos JM, Fahd S (2004) Body size, diet and reproductive ecology of Coluber hippocrepis in the Rif (Northern Morocco). Amphibia-Reptilia 25:287–302
Pleguezuelos JM, Feriche M (2014) Hemorrhois hippocrepis (Linnaeus, 1758). In: Salvador A (ed) Reptiles, 2nd edn, Fauna Ibérica, vol 10. Museo Nacional de Ciencias Naturales, CSIC, Madrid, pp. 723–739
Pleguezuelos JM, Moreno M (1990) Alimentación de Coluber hippocrepis en el SE de la Península Ibérica. Amphibia-Reptilia 11:325–337
Pleguezuelos JM, Fernandez-Cardenete JR, Honrubia S, Feriche M (2007) Correlates between morphology, diet and foraging mode in the Ladder Snake Rhinechis scalaris (Schinz, 1822). Contrib Zool 76:179–186
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/
Salvador A (1984) A taxonomic study of the Eivissa wall lizard, Podarcis pityusensis Boscá 1883. In: Kuhbier H, Alcover JA, Guerau d’Arellano Tur C (eds) Biogeography and ecology of the Pityusic Islands. Springer Netherlands, The Hague, pp. 393–427
Salvador A (2014) Podarcis pityusensis (Boscá, 1883). In: Salvador A (ed) Reptiles, 2nd edn, Fauna Ibérica, vol 10. Museo Nacional de Ciencias Naturales, CSIC, Madrid, pp. 589–601
Salvador A, Pérez-Mellado V (1984) The amphibians and reptiles of the Pityusic Islands. In: Kuhbier H, Alcover JA, Guerau d’Arellano Tur C (eds) Biogeography and ecology of the Pityusic Islands. Springer Netherlands, The Hague, pp. 429–439
Schwenk K (1995) Of tongues and noses: chemoreception in lizards and snakes. Trends Ecol Evol 10:7–12
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Silva-Rocha I, Salvi D, Sillero N, Mateo JA, Carretero MA (2015) Snakes on the Balearic Islands: an invasion tale with implications for native biodiversity conservation. PLoS One 10:e0121026
Simberloff D, Martin JL, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Strauss SY, Lau JA, Carroll SP (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett 9:357–374
Thoen C, Bauwens D, Verheyen RF (1986) Chemoreceptive and behavioural responses of the common lizard Lacerta vivipara to snake chemical deposits. Anim Behav 34:1805–1813
Van Damme R, Quick K (2001) Use of predator chemical cues by three species of lacertid lizards (Lacerta bedriagae, Podarcis tiliguerta, and Podarcis sicula). J Herpetol 35:27–36
Van Damme R, Bauwens D, Thoen C, Vanderstighelen D, Verheyen RF (1995) Responses of naive lizards to predator chemical cues. J Herpetol 29:38–43
Vermeij GJ (1994) The evolutionary interaction among species: selection, escalation, and coevolution. Annu Rev Ecol Evol S 25:219–236
Webb JK, Du WG, Pike DA, Shine R (2009) Chemical cues from both dangerous and nondangerous snakes elicit antipredator behaviours from a nocturnal lizard. Anim Behav 77:1471–1478
Acknowledgments
This work was supported by the Spanish Ministry of Science and Innovation (project CGL2012-39850-CO2-02) and the University of Salamanca (predoctoral grants to AM and ZO). We thank the city council of Sant Josep de Sa Talaia (Ibiza) for providing us accommodations and research facilities at Sa Casilla. We thank A. Pérez-Cembranos for her assistance with the capturing of lizards and Mario Garrido, Gonzalo Rodríguez and Alicia León for their support with the writing and M.T. Mencía and Joseph McIntyre for language revision. We also thank Axios Review, Thomas Madsen and two anonymous reviewers that helped to improve the first version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Spanish Ministry of Science and Innovation (project CGL2012-39850-CO2-02) and the University of Salamanca (predoctoral grants to AM and ZO).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and institutional guidelines for the care and use of animals were followed. Lizards were sampled under permits issued by the Balear Government. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was not required.
Additional information
Communicated by T. Madsen
Zaida Ortega and Abraham Mencía are cofirst authors.
Rights and permissions
About this article
Cite this article
Ortega, Z., Mencía, A. & Pérez-Mellado, V. Rapid acquisition of antipredatory responses to new predators by an insular lizard. Behav Ecol Sociobiol 71, 1 (2017). https://doi.org/10.1007/s00265-016-2246-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-016-2246-4