Abstract
Balancing the costs and benefits of emitting a mating signal is complex. There are direct costs, such as time taken away from essential activities like feeding, and indirect costs, such as attracting unintended receivers, who may gain advantageous information from eavesdropping. As a consequence, the signaler may reduce his chances of mating if the costs outweigh the benefits. Male humpback whales (Megaptera noveangliae) sing. Although it is still unclear whether this signal is aimed at males, females, or both, it is generally accepted this is a breeding signal emitted in the presence of females. This study tested the hypothesis that the cost of singing in humpback whales is attracting other competitors to the group and therefore reducing the chances of the singer successfully mating. Males should therefore “choose” to sing only if the social conditions are not “costly.” We found that males were less likely to sing when joining another group if there were two or more adults already in the group (other competitive males), if there was another singing male in the area (a known competitor), and in higher group densities (resulting in an increased likelihood of having another group, and therefore other competitive males close by). This result was confirmed in later years, where the density of whales had increased substantially but the probability of a male singing while joining other animals had reduced. These groups were more likely to be joined by additional animals in higher density, “riskier” conditions, especially if they included a whale that had joined the group previously while singing. It seems, therefore, that male humpback whales will join other animals while singing when the ‘audience’ comprises of fewer competitive males to reduce the potential cost of attracting eavesdroppers to the group.
Significance statement
The likelihood of a male joining a group while singing was tested against within-group dynamics (number of additional competitive males, presence of a calf), the presence of a nearby singing whale, the nearest neighbor, and the number of groups in the area (group density). Whales were more likely to join groups while singing if there were no other competitive males in the group and no calf, if there were no other singing whales in the area, and if group density was low. In later years, group density substantially increased, resulting in a decrease in the likelihood of a whale joining a group while singing. Results suggest male humpback whales are monitoring their audience composition (surrounding conspecifics) and using this information to make “vocal decisions” when joining a group containing a female.
Similar content being viewed by others
Introduction
Sexual signals, such as song, usually have direct costs to the signaler, and these costs provide reliable information on the quality/traits of the signaling individual (Grafen 1990). Examples of these costs include spending less time on essential activities such as feeding (Cowles and Gibson 2015) or energetic costs (Ward et al., 2003). Several signalers and receivers sharing the same active space constitute a communication network, and this social environment adds additional selection pressures on both signalers and receivers. For example, social eavesdroppers within this network may take advantage of information gained by eavesdropping to increase their own mating success (Dabelsteen et al. 1997; Peake et al., 2001; Peake et al., 2005; Searcy and Nowicki 2005; Clark et al. 2012). This could be viewed as an additional indirect consequence of signaling, as it potentially reduces the signaler’s mating success (Balsby and Dabelsteen 2005). On the other hand, eavesdropping behavior can be especially advantageous to the eavesdropper if one of the main limitations to mating success is finding a receptive mate. Any information that helps to reveal the location of a potential mate (such as eavesdropping on another male’s acoustic sexual display if it might indicate the presence of a female) can increase the chances of a successful courtship and decrease the time spent searching (Waas 1988; Doutrelant and McGregor 2000; Milner et al. 2010). In addition, eavesdroppers can also reduce their cost of displaying by only initiating sexual displays whenever a rival’s display is detected, if it is likely to indicate the presence of a receptive female (Clark et al. 2015). This facilitation of courtship as a consequence of social eavesdropping is therefore thought of as a form of mate competition (Zajonc 1965; Farr 1976; Waas 1988; Doutrelant and McGregor 2000).
Game theory is usually applied to male-male contests to predict the outcome of agonistic interactions based on contest resolution rules (Maynard Smith and Price 1973). Choosing a behavioral tactic, such as displaying, or choosing the amount of time to invest in the behavior (displaying), can also be thought of as “decision making” based on a set of “rules.” Various studies have shown that animals may be monitoring the composition of their audience (surrounding conspecifics) and using this information to make “vocal decisions” (Townsend et al. 2008 and Slocombe et al. 2010: chimpanzees Pan troglodytes; Ung et al. 2011: canaries Serinus canaria; Townsend et al. 2012: meerkats Suricata suricatta; Hedwig et al. 2015: Gorilla gorilla gorilla). Therefore, the “choice” to engage in display tactics (such as vocal signaling) should, to some extent, follow “rules” based on the signaler’s social environment, such as the availability of receptive females, the number of other males (competitors) in the area, and/or the quality of these other males (the composition of the “audience”).
Humpback whales use “song” as a display tactic. Songs are relatively loud and are likely to be detectable by conspecifics over distances of tens of kilometers (Au et al. 2006) resulting in a large communication space. The exact function of song in humpbacks is still debatable but has been hypothesized to be a means of sexual advertisement directed towards the female (Winn and Winn 1978; Tyack 1981; Chu and Harcourt 1986; Smith et al. 2008), for male dominance sorting (Darling and Bérubé 2001; Darling et al. 2006), as a threat display during intra-sexual competition (Baker and Herman 1984), to mediate aggressive or cooperative interactions (Winn and Winn 1978; Tyack 1981; Darling et al. 2006), or for mediating spacing between singers (Frankel et al. 1995). Whatever its function, it is generally accepted that song in humpback whales functions as a breeding display; therefore, the “decision” to sing (like the decision to compete), as well as how much time to invest in singing, should follow a set of “rules” based on the costs versus benefits of displaying.
The breeding behavior of male humpback whales is highly variable. Males may sing for tens of minutes to several hours and often stop singing before they actively join with other whales (Tyack 1981; Tyack and Whitehead 1983; Baker and Herman 1984; Darling and Bérubé 2001; Darling et al. 2006). Others continue to sing after they have joined (Darling et al. 2006; Smith et al. 2008). Regardless of who joins whom, males do not usually sing when associating with other males, but do sing when associating with females (Smith et al. 2008). Only some of the males in a population sing at any time. Other non-singing whales can join singing whales (usually other males) whereupon they usually stop singing (Darling and Bérubé 2001; Darling et al. 2006). Non-singing males also commonly join and escort females with a newborn calf, and the frequency that this occurs increases as the breeding season progresses, at least partly due to there being increasing numbers of neonates (Craig et al. 2003; Morete et al. 2007). Although not singing, these joining whales may use other acoustic signals known as “social sounds” (Dunlop et al. 2008). These sounds include the sounds from energetic surface behaviors (breaches, pectoral slapping, tail slapping; Dunlop et al. 2010) and single or small groups of vocalizations that have very limited serial patterning and occur in short bursts (Dunlop et al. 2007; Rekdahl et al. 2015). Although earlier work assumed these sounds were produced only in aggressive and/or competitive social encounters (Tyack 1983; Tyack and Whitehead 1983; Baker and Herman 1984; Silber 1986), later studies found that they are used in various other social and behavioral contexts including by females and calves (Dunlop et al. 2008). Part of the social sound repertoire includes “song-unit social sounds” which are sounds that are also heard in contemporary song, but not “sung” in a long, continuous, and repetitive way (Dunlop et al. 2008). Social sounds are likely to be audible to other groups in the area (though at much shorter distances compared to song; Dunlop et al. 2013) allowing groups to monitor their social environment.
Generally, a highly skewed operational sex ratio (where there are more males than sexually receptive females) predicts there should be greater competition among males to gain access to these sexually receptive females (Trivers 1972; Andersson 1994; Wade et al. 2003). In the southern hemisphere, during the southward (poleward) migration of humpback whales, the operational sex ratio is likely to be highly skewed towards males. Many females will have just given birth, or become pregnant, further north in the breeding grounds and although postpartum estrus is possible in humpbacks, the incidence (or period that estrus lasts) may be low (Chittleborough 1958). Therefore, males in an area are likely to be in competition with each other to gain access to the few remaining receptive females. This gives the opportunity to test the potential effects of the “audience” on broadcast signaling behavior in a communication network setting, where the “audience” is comprised of potential competitors. Visual and acoustic observations of joining animals during the southward migration of humpback whales (off the eastern coast of Australia) determined that some whales joined others while singing (Smith et al. 2008) while some others used social sounds (Dunlop et al. 2008). Some animals were also found to switch between vocal strategies; first they joined a group while singing, stopped singing, and split from the group, then joined a different group emitting social sounds (RAD 2008, unpubl. obs.). This implies that male humpbacks whales do not exclusively adhere to using one signal type (song or social sounds) when joining another group, but may switch from one strategy to another depending on the prevailing social conditions.
A previous study showed that humpback whale groups change their social sound signals in the presence of an audible bystander (a singing whale) by reducing their vocal levels (Dunlop 2016): an “audience effect.” The current study will test the effect of the “audience” on the singing behavior of other males. Using observational data, we will test the hypothesis that the “decision” to sing when joining other groups, as well as the time invested in singing, follows a set of “rules” and these “rules” function to reduce the associated cost of singing within a communication network. Given that the cost of singing is unlikely to be energetic due to the large body size of the whales, the study will assume a large proportion of the cost is related to the social environment, in that more animals in the area equates to more eavesdroppers and therefore more potential competitors. Over the course of the study, this humpback whale population consistently increased at a rate of 10.9 % per annum (95 % CI 10.5–11.3 %) resulting in an approximately 2.5-fold increase in population between 2002 and 2011 (Noad et al. 2011). This provides an opportunity with which to test for an effect of a general increase in population size and density on their signaling behavior, given that this is likely translated to an increase in male competitors. We will also assume that vocalizing joining whales will use one of two strategies: song or social sounds and that the underlying preference is for males to sing (display) when joining rather than not sing, as long as the benefits outweigh the costs.
Methods
Visual and acoustic data collection
The eastern Australian population of humpback whales migrates annually along the eastern Australian coastline between feeding areas in the Antarctic and breeding grounds inside the Great Barrier Reef off central Queensland. During migration, they pass close to shore in the vicinity of Peregian Beach, 130 km north of Brisbane, where this study was conducted. Data were collected as part of the Humpback whale Acoustic Research Collaboration (HARC) project during the southward migration in September/October 2002, 2003 and 2004 (for detailed methods, see Noad et al. 2004) and during the Behavioral Response of Australian Humpbacks to Seismic Study (BRAHSS) in 2010 and 2011. The BRAHSS study involved experiments with air guns; however, there were no air gun experiments during the observations included in this study.
Land-based observations (including the position, composition, and behaviors) of all migrating groups passing through the study area were collected daily (7 am to 5 pm, weather permitting) from an elevated survey point, Emu Mountain (73 m elevation). A theodolite (Leica TM 1100) was used in conjunction with a notebook computer running VADAR software (E. Kniest, Univ. Newcastle, Australia) to track the groups in real time. Each theodolite fix was time-stamped and the behavior of the fixed whale (e.g., blow or surface behaviors such as breach, pectoral flipper slap, tail slap, etc.), group composition, direction of travel, and any other notes of interest (e.g., splitting or joining of groups) were recorded with each fix. Other additional observations were made using binoculars. These observations were also recorded onto VADAR (in real time). Weather was noted hourly and observations included sea state and wind speed cloud cover, glare strength and position, swell height and direction, and rainfall.
Acoustic recordings were made from three to five hydrophone buoys moored in 18–28 m of water and arranged in a line or T-shaped array. Each hydrophone buoy consisted of a surface buoy containing a custom-built pre-amplifier (+20 dB gain) and 41B sonobuoy VHF radio transmitter. A High Tech HTI-96-MIN hydrophone with built-in +40 dB pre-amplifier was suspended approximately 1 m above each buoy’s mooring, and its cable ran up the anchor rope to the buoy where it connected to the pre-amplifier and transmitter. Buoys 1–3 were approximately 750 m apart and were arranged in a line parallel to, and 1.5 km from, the beach. Buoys 4 and 5 were moored seaward from buoy 2 approximately 600 m apart, so that buoys 2, 4, and 5 formed a line perpendicular to that of buoys 1–3. Signals were received onshore at a base station 1.5 to 2.5 km away using a directional Yagi antenna and type 8101, four-channel sonobuoy receiver. This was connected to a PC and acoustic data were recorded to hard disk via a series E National Instruments Data Acquisition Card and recorded using Ishmael acoustic tracking software (D. Mellinger, Oregon State Univ.) usually at a sampling rate of 22 kHz (per channel), 16-bit depth.
Ishmael was also used to determine the location of sound sources detected. This was achieved by cross-correlation of the same sound arriving at the different hydrophones to determine differences of the arrival time of the sound at the buoys. These differences, together with an accurate knowledge of the positions of the hydrophones, were then used to determine the most likely location of the source (e.g., singing and vocalizing whales). Small errors in determining the time of arrival differences can result in errors in the distance measurements to the source (although the bearing is usually robust). However, sound location accuracy was significantly improved by taking the mean position of several estimates over a brief period and by using more than three buoys (Noad et al. 2004).
Group composition and group social behavior
Behavioral analyses were performed post-field; therefore, observers were blind to the aims and analysis methods during the data collection phase. All joining interactions (see Table 1 for the definition of a joining interaction) were isolated from the VADAR data files and each interaction was labeled as either a “singer join” (and given a 1) or “non-singer join” (and given a 0, Table 1). Within “singer joins,” the time spent singing was also noted (including before, during, and after the sighted join).
The composition of groups was also recorded before and after the join. Groups commonly seen in the study area included adult only groups containing from one to three whales, female-calf groups (an adult with a calf; the adult presumed to be the mother of the calf), and female-calf groups with one or more “escorts” (other adults which are presumed to be males; Tyack 1981; Tyack and Whitehead 1983; Baker and Herman 1984) which were called “female-calf-escort” and “female-calf-multiple escort” groups. Adults in some of these groups can be singers: a one singer, a singer accompanied by another single whale, and a female-calf group with a singing escort. In the singer join interactions included in this study, the lone singing whale was always the joiner (interactions in which the singer was joined by another animal were excluded from the analysis). Interactions where female-calf groups joined each other (more common in the 2010–2011 dataset) were excluded. Based on the remaining group compositions, joining interactions were divided in to three categories, effectively the composition of the group before the join (Table 2).
Any more animals that joined the group were also noted, as well as whether or not the group split after joining. Further joins to the group were not counted as separate interactions and so all initial joining interactions were assumed to be independent samples. If an animal (or group of animals) joined a group, split from that group, and then joined a second group, only the first joining interaction was included in the analysis.
The amount of time the group was in visual range of the land-based station after the join was also noted (ranging between 10 min and 4 h). The time taken for an additional animal to join the group (if this occurred) was also noted (ranging from 10 to 80 min with most of the joins occurring before 40 min). Given that the probability of observing, or not observing, another animal joining the group was, to some extent, dependent on the time the group remained within visual range, some correction had to be made based on how much time the group was in visual range after the initial join. Groups that remained in visual range for more than 40 min, and had no observed further joins during this time, were given a probability of an additional join of 0. Groups that had no sighted further joins, but were in visual range for less than 40 min, were eliminated from this part of the analysis. All remaining groups (which had at least one sighted additional join) were given a probability of 1.
Social environment
From the acoustically tracked singer data, the presence of a singing whale at the time of joining was noted (or noted as “none” if there were no acoustically tracked whales in the area or only very faint song) as well as the distance of the closest singing whale from the joining group. These data were categorized to “within 5 km” and “beyond 5 km” from the joining group. The distance of the closest group (nearest neighbor) was categorized to within 2.5 km, between 2.5 and 5 km, and beyond 5 km, and the composition of this group was also noted. Finally, the number of groups within 5 km of the joining group (at the time joining was observed) was also counted.
Social vocalizations in joining groups
In the 2002–2004 dataset, 32 joining groups were within the detection limits of the acoustic array while joining (Dunlop et al. 2013) and had concurrent acoustic recordings available. Out of these, non-song social vocalizations were heard in 30 groups. In the 2010–2011 dataset, because of the different settings in the recording system, only 17 groups were within the detection limits of the array during joining. Of these, social vocalizations were audible in only five groups (sounds from 11 of the “inaudible” groups were likely masked by a small research vessel undertaking behavioral observations at the time of the study). However, in six joining groups where social vocalizations were not heard, the join occurred within the detection limits of the array and there was no research vessel following the group. Therefore, these groups either remained silent during the joining interaction, or emitted vocalizations at very low, undetectable levels. Considering this, the sample size for 2010–2011 was only 11 (five audible and six “silent”), giving a total sample size of 43 joining groups that were silent (8) or used social sounds (35) during joining for the full dataset.
Recordings of non-song social vocalizations that were tracked acoustically to either the joining animal or groups being joined from around the time of the join (defined as from 10 min before the join to 10 min after) were analyzed further. Vocalizations were qualitatively and quantitatively classified into sound types based on earlier social sound studies (Dunlop et al. 2007; Rekdahl et al. 2013). Sound types were then re-categorized into “song-unit” social vocalizations (Dunlop et al. 2007) and “non-song stable” vocalizations (Rekdahl et al. 2013). Typical “non-song stable” vocalizations heard during both singer and non-singer joins were “thwops,” “wops,” “snorts,” “grumbles,” and “grunts” (see Dunlop et al. 2007 for full sound description). These are the most common vocalizations used by this population of humpback whales during migration (Dunlop et al. 2007), are part of the vocal repertoire every year (Rekdahl et al. 2013), and are not part of the song repertoire. In singer joins, sounds that were acoustically tracked were from the group being joined by the singer (before joining), never the singer. In non-singing joins, most of these vocalizations, except the “thwops,” were also acoustically tracked to the group being joined. Most “thwops” that could be tracked came from the joining whale.
In the 2002–2004 dataset, there were 21 other vocalizations heard exclusively in non-singer joins (from seven joining groups). Out of these, 20 were units from 2002, 2003, or 2004 song (the other being a high-frequency blowhole-associated sound called a “scream” [Dunlop et al. 2007]). These song units sometimes occurred in bouts similar to song phrases and were acoustically tracked to the joiner, rather than the group being joined (before joining was observed). “Song-unit social sounds” are not stable over time in that they appear and disappear from the social sound repertoire as they appear and disappear in the song (Rekdahl et al. 2013). In the 2010–2011 dataset,“song-unit social sounds” were only heard in one group (10 sound types which were also heard in the 2010/2011 song).
Joining interactions in which social vocalizations were heard were then classified as “non-song stable” (no “song-unit social sounds” heard) or “song-unit” (“song-unit social sounds” heard in addition to “non-song stable” sounds). Given the small sample size, only summary statistics will be presented.
Statistical analysis
All analyses were conducted using the statistical software package “R” (R Core Team 2012). Logistic regression models were used to test the probability of (1) an animal joining a group as a singing whale (P) and (2) the probability of an additional animal joining the group after the initial join (J). Predictor variables included in the models were (1) the presence of another singing whale (within and beyond 5 km), (2) the distance of the nearest neighbor (within 2.5, 2.5–5, and beyond 5 km) ,and (3) the number of other groups within 5 km. Response variables took on values of 0 or 1 resulting in a binomial distribution. For models testing the effects of various predictor variables on the time spent singing (T), the Gamma distribution was used (due to the non-normality of the data).
The full dataset was split into two parts: 2002–2004 (86 joining interactions after excluding eight where a singer was joined by another animal and one where two female-calf pairs joined) and 2010–2011 (88 joining interactions after excluding three where the singer was joined by another animal and 19 where two female-calf pairs joined). This allowed us to account for, and test for, an effect of increased density of whales over time. First, a model was developed using the 2002–2004 dataset. A separate model was then developed using the 2010–2011 dataset, to test if the same variables dictated whether or not a whale joined a group while singing (P) and the time spent singing (T). Finally, the full dataset was analyzed and the effect of “year” (either 2002–2004 or 2010–2011) included as an additional predictor variable. Only the full dataset was analyzed to assess the effect of various predictor variables in the probability of an additional animal joining the group (J).
Models, using various combinations of predictor variables, were compared using likelihood ratio tests with the chi-squared (χ 2) distribution to calculate p values. Within-model binomial effects are presented as z values with associated p values (significance was set at p < 0.05). Within-model effects of Gamma distribution models are presented as t values with associated p values (significance was set at p < 0.05). To generate effect sizes, predictions for each response variable were calculated based on the model output.
Results
Singer versus non-singer joins
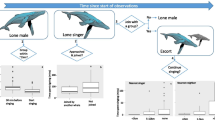
The model was first created using the 2002–2004 dataset. The best model (Table 3) to predict the probability of joining a group as a singer (in order of deviance explained) was the presence of another singing whale in the area, the animals involved in the join (Fig. 1), the number of groups within 5 km of the joining group (Fig. 2), and the distance of the nearest neighbor. Single adults and females with a calf were significantly more likely to be joined by singing whales (z = 3.52, p = 0.004 and z = 3.51, p = 0.004) compared to groups already containing more than one adult (Fig. 1). In terms of the social environment, there were no instances of a singer joining a group of any type when there was another singer within 5 km of the joining group (Fig. 1). In addition, whales were less likely to join as a singer with a greater number of groups within 5 km (z = −2.45, p = 0.014; Fig. 2) and if there was a nearest neighbor within 2.5 km of the group (z = 1.41, p = 0.159; Fig. 2). Though this latter result was not a significant within-model effect, there was a highly significant relationship between the number of groups in the area and the distance of the nearest neighbor (within 2.5 km and 2.5–5 km) from the joining group (p < 0.0001). In other words, the more groups in the area, the more likely the joining group had another group close by.
The predicted probability (including median, first and third quartile, and minimum and maximum values) of an animal joining a group while singing for each joined group composition (Table 2) using the 2002–2004 data. The upper graph is in the presence of another singing whale (within 5 km) and the lower graph is in the absence of a singing whale within 5 km
Illustrating the relationship (using predicted values) between the probability of a whale joining a group while singing and the number of other groups within 5 km of the joining group. The solid line represents joining whales in the presence of another singing whale (within 5 km) and the shaded area (including the three dashed lines representing the 3 social compositions) represents joining whales when there was no singer within 5 km. The upper plot is the 2002–2004 and the lower plot is the 2010–2011 data
Analysis of the 2010–2011 dataset revealed that the best explanatory model included the same variables as the 2002–2004 dataset. However, there were no significant within-model effects. In 2002–2004, only 18 (out of 86) groups had a singer within 5 km while joining compared to 30 (out of 88) groups in 2010–2011. There was also an increase in number of groups within 5 km between the two timeframes, with 0–6 groups (average of 2) in 2002–2004 compared to 0–10 (average of 4) in 2010–2011. With this increase in the likelihood of having a singer within 5 km, and the increase in nearby whales, predictions from the 2002–2004 suggests there should be a substantial decrease in the number of singer joins in 2010–2011. In fact, only 10 out of 88 joins involved singers in 2010–2011 (and the probability of joining as a singer was only 0.1) compared to 40 out of 86 joins in the 2002–2004 dataset (where the probability of joining as a singer was 0.3; Fig. 2). Including “year” as an additional effect (in the full dataset) showed that whales were significantly (z = −3.10, p = 0.002) less likely to join as a singer in 2010–2011 (Table 4), even when controlling for the presence of a singing whale and the number of other groups in the area (Fig. 2). In other words, even when there were few other groups in the area, the probability of a whale joining as a singer in these later years was still relatively low compared to the 2002–2004 years (Fig. 2).
Time spent singing
Only joins involving singing whales were used for this analysis (n = 48). The total time spent singing (before and after the join) ranged from 6 to 234 min and (in both the 2002–2004 and 2010–2011 datasets) was not significantly related to the singer’s social environment (any of the predictor variables). In the full dataset, “year” was the only significant effect as singing whales sang for significantly longer in 2010–2011 compared to 2002–2004 (t = 2.26, p = 0.03). Therefore, although fewer whales joined as singing whales in 2010–2011, the whales that did sing invested more time in singing (Fig. 3).
The “cost” of singing
In this study, we assumed a potential “cost” of singing was that other males might be attracted to the group, which was quantified as another animal, or animals, joining the group (within 40 min of the original join). Groups were more likely to be joined by more animals when there were more groups in the area and when there was a nearest neighbor close by (Table 5), though neither included variable was significant within the model. The composition of the group being joined and the type of join (singer or non-singer) did not explain much deviance. However, this is to be expected if most whales followed the “rules” outlined above, resulting in a low incidence of more animals being attracted to the group.
To test if whales that followed the “rules” were less likely to attract another animal to the group, and if this likelihood was reduced by not singing while joining, we created two “rule” categories. “Rule-breakers” were those that joined when the number of groups within 5 km was greater than 3 and/or there was a nearest neighbor within 2.5 km. The remaining joiners categorized as “rule-keepers.” Within these two categories, joiners were further separated into “singer” and “non-singer.” The analysis model (Table 6) results suggest that “rule-breakers” were more likely to have more animals joining their group, but this was also dependent on whether or not they sang. “Rule-breaker” singers were 42 % more likely to have more animals joining compared to “rule-keeper” singers (there were no instances of “rule-keeper” singer groups being joined by another animal). However, “rule-breaker” non-singers were only 16 % more likely to have more animals joining the group (Fig. 4). In other words, even if the joining animal did not sing, there was still a low chance of these groups being joined by more animals, but singing “rule-breakers” had the highest chance of attracting more (likely) males.
The alternative to singing
A total of eight groups within the detection limits of the array did not produce audible social sounds (in the full dataset). Interactions consisted of a singer joining a female-calf (4), a singer joining another adult (1), and a non-singer joining a female-calf (3). Out of the 35 audible groups, 19 involved a joining singing whale and “non-song stable” vocal sounds were tracked to the group being joined. “Song-unit social sounds” were never heard from these groups.
The remaining 16 audible groups involved a join by a non-singer (8 using “stable” only and 8 using “song-unit” social vocalizations). Interestingly, the majority (75 %) of groups that used “song-unit social sounds” involved a non-singing adult joining a group containing two or more adults (Table 7). “Non-song stable” vocalizations were also heard in these interactions (usually from the group being joined) but many of the “song-unit social sounds” were acoustically tracked to the joiner, suggesting an alternate joining signal to song. Most joins using “non-song stable” vocalizations only involved adult joining a female-calf (75 %). Some of the sounds (“thwops”) were acoustically tracked to the joiner, suggesting joining animals (especially single animals joining a female-calf pair) may also use certain “stable” vocalizations when joining instead of song.
Discussion
The results suggest that whether or not a male humpback whale joins another group while singing is dependent on the social composition of the group being joined as well as the social environment (the presence of another singing whale, the number of other groups in the area, and the distance of the nearest other group). Males were less likely to sing when (a) joining two or more adults (using “song-unit social sounds” instead), (b) if there was another singing male in the area, and (c) in higher group densities (resulting in an increased likelihood of having another animal or group of animals nearby). In later (higher density) years, although the probability of a male singing while joining followed the same “rules” as in the lower density years, the overall probability of a male singing while joining had declined. In a male-male competition environment, if the audience (presence of eavesdroppers) has high costs to signallers (Balsby and Dabelsteen, 2005), then signalers should adjust their behavior towards their opponent to conceal information (Evans and Marler 1994; Doutrelant et al. 2001; Peake et al. 2001; Matos and McGregor 2002). In this study, we assumed the “cost” of singing was the attraction of other eavesdropping male competitors, quantified as the probability of more (likely) males joining the group (more likely to happen in higher density conditions and with close-by neighbors). Our results suggest that, as with these previous studies, humpback whale signalers, in a communication network setting, account for their immediate social environment (audience) by adjusting their signaling behavior to reduce the potential cost of displaying.
With the large increase in population over the course of the study, joining groups had a higher chance of having singing males in the area, the number of groups around the joining group increased, and there was a greater chance of having a close-by nearest neighbor. Given this change in social environment, the results in 2002–2004 suggest that humpback whales should join less often as a singing whale given the increase in “risk” from additional male competitors. This is in fact what was observed. In the 2002–2004 dataset, the probability of joining a group as a singer was 0.3, compared to only 0.1 in 2010–2011, despite the fact that the sample size of joins in both datasets was about the same. However, even when controlling for the immediate social environment, there was still a significant effect of “year” suggesting an additional effect not accounted for in this analysis. This effect is likely to be related to the broader social environment. Male whales potentially have access to many cues about the general density of the population, particularly the numbers of other singers that they hear hour-to-hour, day-to-day, across the breeding season. This may be sufficient to increase their perception of risk and so reduce the likelihood of joining using song. The longevity of this dataset, and the fact that this humpback whale population is increasing at such a rapid rate, has provided a unique opportunity with which to test population-level effects on signaling behavior within a communication network. The results show that these animals adapt to a changing social environment (due to changes in population density) and illustrate the plasticity of communication strategies.
Interestingly, whales that did sing during a joining interaction in 2010–2011 sang for longer (though this was based on a relatively small sample size). It appears that in an increasingly competitive environment (higher density of animals), fewer animals take the “risk” of singing when joining a group, even if the immediate social conditions favor singing. However, those that did sing sang for longer. In some species of lekking birds, display “effort” (e.g., the length of the display or number of display calls) is correlated with mating success (Fiske et al. 1998; McDonald 1989; Alonso et al. 2010). Perhaps in an increasingly competitive environment, only the “risk-takers” will sing, and will sing for longer, which may provide some information on the quality of the male. This could support a multi-function interpretation of song. Males use song to facilitate joining with females, suggesting a role in courtship (Smith et al. 2008) while also demonstrating to rival males that they are willing to display in “riskier” conditions. However, the appraisal of the singer as a risk-taker by nearby rival males depends on them being aware that the singer has joined a female. Whether this is the case is not clear.
An important predictor of joining strategy in this dataset was the social composition of the group being joined: another adult, a female with a calf, or a group already containing two or more adults. During the breeding season, multiple adult groups are usually competitive, consisting of a female with multiple male escorts competing for access to her (Tyack 1983; Baker and Herman 1984). There is a general rarity in female associations (Medrano et al. 1994; Brown and Corkeron 1995; Clapham 2000); therefore, the composition of interacting groups can be predicted. In this study, whales rarely joined groups already containing two or more adults (probably consisting of one female and one or more males) using song, irrespective of whether another singer was singing in the area. This is consistent with observations of previous studies, which found that singing whales stopped singing before joining competitive groups (Tyack 1981; Frankel et al. 1995). These joining males may be investing more time in attempting to physically out-compete other males already in the group to gain access to the female, rather than investing time in singing. While engaged in the active pursuit of, and competition for, a female, investing time in song is unlikely to be advantageous. Therefore, this cessation in song may not be an anti-eavesdropping strategy, but a complete switch in mating strategies. Clapham (2000) suggested three primary mating strategies in male humpback whales: (1) singing (displaying) to attract females, (2) escorting an estrus or proestrus female, and (3) direct competition with other males. Studies in fish (Poecilia mexicana) have concluded that the immediate social environment (the presence of a conspecific) can affect the expression of mating strategy (Plath and Strecker 2008). Primates have also been shown to call “strategically” based on the social composition of the audience to minimize risks from competition (Townsend et al. 2008), maintain important social bonds (Slocombe et al. 2010), and reinforce their social position within the group (Laporte and Zuberbühler 2010). In fact, in this study, whales joining multiple adult groups were also more likely to emit “song-unit social sounds,” implying that these animals are using a different vocal strategy to song. “Song-unit social sounds” are song-like sounds, though emitted at lower source levels to song (Dunlop et al. 2013) and are usually comprised of a short bout of song units, rather than a continuous song. Perhaps these signals have a similar function to song but are less likely to attract other males given their lower level and shorter duration.
Lone singing whales (“display singing”) that were joined by other whales were not included in this study (Clapham 2000’s mating strategy 1). Escorting animals (joiners), in this study, were highly likely to join a single adult using song, and about 50 % of the time, joined a female with a calf using song. Given that males do not sing when in a group with other males (Darling and Bérubé 2001; Smith et al. 2008) but do sing when associating with females (Smith et al. 2008), the single adults joined using song were likely to be females. Although, in this study, there is no available information on the state of estrus of the females within these groups (a method for determining estrus in live humpback whales has not yet been developed), it is likely that this also plays a role in determining the vocal behavior of the joining males. Male Siamese fighting fish (Betta splendens) alter their signaling behavior based on, among other things, the reproductive state of the female and the sex of the “audience” (Doutrelant et al. 2001; Matos and McGregor 2002; Dzieweczynski et al. 2005). Similarly, male humpback whales may be more willing to “risk” singing in the presence of a female in estrus (adult without a calf) compared to a female with a calf (less likely to be in estrus). Therefore, in humpback whales, there seems to be complex inter-relationship between the immediate social environment (within the group) and the broader social environment (other groups and singing whales in the area) in determining their mating strategy and display tactics. It seems that not only “display” singing comes at a cost, but continuing to sing as an escort also can be “risky” in that it attracts other males, meaning the singer has lost exclusive access to a female.
Signaling strategies in humpback whales are therefore highly dependent on their social environment. However, further work should include information on the physical attributes of the singing males, including their size, physical body condition, and age, to look for evidence of tactical song use by a particular cohort. Examining use of song during joining on the breeding grounds themselves would also be of interest to see if this is influenced by social environment in the same way as during migration, or whether different rules apply. Similar studies in primates have interpreted these findings as animals making cognitive vocal decisions based on their receiver and their audience (Slocombe and Zuberbühler 2007; Zuberbühler 2008). It seems that singing humpback whales also closely follow a number of “rules,” though whether or not this is cognitive, and whales tactically control their “decision” to sing, requires further study. Interestingly, vocalizing humpback groups (those producing non-song social sounds) also change their vocal behavior according to the audience composition (nearby singing whales) by lowering their vocal amplitude (Dunlop 2016). This suggests some form of tactical decision making, as presumably these groups are vocally avoiding a known male in the area. As this area is very new in baleen whale communication, how much cognition plays a part in these vocal “decisions” remains to be seen.
References
Alonso JC, Magana M, Palacin C, Martin CA (2010) Correlates of male mating success in great bustard leks: the effects of age, weight, and display effort. Behav Ecol Sociobiol 64:1589–1600
Andersson S (1994) Costs of sexual advertising in the lekking Jackson widowbird. Condor 96:1–10
Au WWL, Pack AA, Lammers MO, Herman LM, Deakos MH, Andrews K (2006) Acoustic properties of humpback whale songs. J Acoust Soc Am 120:1103–1110
Baker CS, Herman LM (1984) Aggressive-behavior between humpback whales (Megaptera novaeangliae) wintering in Hawaiian waters. Can J Zool 62:1922–1937
Balsby TJS, Dabelsteen T (2005) Simulated courtship interactions elicit neighbour intrusions in the whitethroat, Sylvia communis. Anim Behav 69:161–168
Brown M, Corkeron P (1995) Pod characteristics of migrating humpback whales (Megaptera novaeangliae) off the east Australian coast. Behaviour 132:163–179
Chittleborough RG (1958) The breeding cycle of the female humpback whale, Megaptera nodosa (Bonnaterre). Aust J Mar Fresh Res 9:1–18
Chu K, Harcourt P (1986) Behavioral correlations with aberrant patterns in humpback whale songs. Behav Ecol Sociobiol 19:309–312
Clapham PJ (2000) The humpback whale. In: Mann J, O’Connor R, Tyack PL, Whitehead H (eds) Cetacean societies. Field studies of dolphins and whales. The University of Chicago Press, London
Clark DL, Roberts JA, Uetz GW (2012) Eavesdropping and signal matching in visual courtship displays of spiders. Biol Lett 8:375–378
Clark DL, Zeeff CK, Sabovodny G, Hollenberg A, Roberts JA, Uetz GW (2015) The role of social experience in eavesdropping by male wolf spiders (Lycosidae). Anim Behav 106:89–97
Cowles SA, Gibson RM (2015) Displaying to females may lower male foraging time and vigilance in a lekking bird. Auk 132:82–91
Craig AS, Herman LM, Gabriele CM, Pack AA (2003) Migratory timing of humpback whales (Megaptera novaeangliae) in the central North Pacific varies with age, sex and reproductive status. Behaviour 140:981–1001
Dabelsteen T, McGregor PK, Holland J, Tobias JA, Pedersen SB (1997) The signal function of overlapping singing in male robins. Anim Behav 53:249–256
Darling JD, Bérubé M (2001) Interactions of singing humpback whales with other males. Mar Mammal Sci 17:570–584
Darling JD, Jones ME, Nicklin CP (2006) Humpback whale songs: do they organize males during the breeding season? Behaviour 143:1051–1101
Doutrelant C, McGregor PK (2000) Eavesdropping and mate choice in female fighting fish. Behaviour 137:1655–1669
Doutrelant C, McGregor PK, Oliveira RF (2001) The effect of an audience on intrasexual communication in male Siamese fighting fish, Betta splendens. Behav Ecol 12:283–286
Dunlop RA (2016) Changes in vocal parameters with social context in humpback whales: considering the effect of bystanders. Behav Ecol Sociobiol 70:857–870
Dunlop RA, Noad MJ, Cato DH, Stokes D (2007) The social vocalization repertoire of east Australian migrating humpback whales (Megaptera novaeangliae). J Acoust Soc Am 122:2893–2905
Dunlop RA, Cato DH, Noad MJ (2008) Non-song acoustic communication in migrating humpback whales (Megaptera novaeangliae). Mar Mammal Sci 24:613–629
Dunlop RA, Cato DH, Noad MJ (2010) Your attention please: increasing ambient noise levels elicits a change in communication behaviour in humpback whales (Megaptera novaeangliae). Proc R Soc Lond B 277:2521–2529
Dunlop RA, Noad MJ, Cato DH (2013) Source levels of social sounds in migrating humpback whales (Megaptera novaeangliae). J Acoust Soc Am 134:706–714
Dzieweczynski TL, Earley RL, Green TM, Rowland WJ (2005) Audience effect is context dependent in Siamese fighting fish, Betta splendens. Behav Ecol 16:1025–1030
Evans CS, Marler P (1994) Food calling and audience effects in male chickens, Gallus-gallus—their relationships to food availability, courtship and social facilitation. Anim Behav 47:1159–1170
Farr JA (1976) Social facilitation of male sexual-behavior, intra-sexual competition, and sexual selection in guppy, Poecilia reticulata (Pisces: Poeciliidae). Evolution 30:707–717
Fiske P, Rintamaki PT, Karvonen E (1998) Mating success in lekking males: a meta-analysis. Behav Ecol 9:328–338
Frankel AS, Clark CW, Herman LM, Gabriele CM (1995) Spatial-distribution, habitat utilization, and social interactions of humpback whales, Megaptera novaeangliae, off Hawaii, determined using acoustic and visual techniques. Can J Zool 73:1134–1146
Grafen A (1990) Biological signals as handicaps. J Theor Biol 144:517–546
Hedwig D, Mundry R, Robbins MM, Boesch C (2015) Audience effects, but not environmental influences, explain variation in gorilla close distance vocalizations—a test of the acoustic adaptation hypothesis. Am J Primatol 77:1239–1252
Laporte MNC, Zuberbühler K (2010) Vocal greeting behaviour in wild chimpanzee females. Anim Behav 80:467–473
Matos RJ, McGregor PK (2002) The effect of the sex of an audience on male-male displays of Siamese fighting fish (Betta splendens). Behaviour 139:1211–1221
Maynard Smith J, Price GR (1973) The logic of animal conflict. Nature 246:15–18
McDonald DB (1989) Correlates of male mating success in a lekking bird with male male cooperation. Anim Behav 37:1007–1022
Medrano L, Salinas M, Salas I, Deguevara PL, Aguayo A, Jacobsen J, Baker CS (1994) Sex identification of humpback whales, Megaptera novaeangliae, on the wintering grounds of the Mexican Pacific Ocean. Can J Zool 72:1771–1774
Milner RNC, Jennions MD, Backwell PRY (2010) Eavesdropping in crabs: an agency for lady detection. Biol Lett 6:755–757
Morete ME, Bisi TL, Rosso S (2007) Temporal pattern of humpback whale (Megaptera novaeangliae) group structure around Abrolhos Archipelago breeding region, Bahia, Brazil. J Mar Biol Assoc UK 87:87–92
Noad MJ, Cato DH, Stokes MD (2004) Acoustic tracking of humpback whales: measuring interactions with the acoustic environment. In: Mee DJ, Hooker RJ, Hillock ID (eds) Acoustics 2004: transportation noise and vibration—the new millenium. Proceedings of the Annual Conference of the Australian Acoustical Society. Australian Acoustical Society, Darlinghurst, NSW, Australia, pp. 353–358
Noad MJ, Dunlop RA, Paton D, Kniest H (2011) Abundance estimates of the east Australian humpback whale population: 2010 survey and update. Paper submitted to the International Whaling Commission Scientific Committee, Tromsø, Norway, SC/63/SH22
Peake TM, Terry AMR, McGregor PK, Dabelsteen T (2001) Male great tits eavesdrop on simulated male-to-male vocal interactions. Proc R Soc Lond B 268:1183–1187
Peake TM, Matessi G, McGregor PK, Dabelsteen T (2005) Song type matching, song type switching and eavesdropping in male great tits. Anim Behav 69:1063–1068
Plath M, Strecker U (2008) Behavioral diversification in a young species flock of pupfish (Cyprionodon spp.): shoaling and aggressive behavior. Behav Ecol Sociobiol 62:1727–1737
R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Rekdahl ML, Dunlop RA, Noad MJ, Goldizen AW (2013) Temporal stability and change in the social call repertoire of migrating humpback whales. J Acoust Soc Am 133:1785–1795
Rekdahl ML, Dunlop RA, Goldizen AW, Garland EC, Biassoni N, Miller P, Noad MJ (2015) Non-song social call bouts of migrating humpback whales. J Acoust Soc Am 137:3042–3053
Searcy WA, Nowicki S (2005) Evolution of animal communication: reliability and deception in signaling systems. Princeton University Press, Princeton
Silber GK (1986) The relationship of social vocalizations to surface behaviour and aggression in the Hawaiian humpback whale (Megaptera novaeangliae). Can J Zool 64:2075–2080
Slocombe KE, Zuberbühler K (2007) Chimpanzees modify recruitment screams as a function of audience composition. P Natl Acad Sci USA 104:17228–17233
Slocombe KE, Kaller T, Turman L, Townsend SW, Papworth S, Squibbs P, Zuberbühler K (2010) Production of food-associated calls in wild male chimpanzees is dependent on the composition of the audience. Behav Ecol Sociobiol 64:1959–1966
Smith JN, Goldizen AW, Dunlop RA, Noad MJ (2008) Songs of male humpback whales, Megaptera novaeangliae, are involved in intersexual interactions. Anim Behav 76:467–477
Townsend SW, Deschner T, Zuberbühler K (2008) Female chimpanzees use copulation calls flexibly to prevent social competition. PLoS One 3:e2431
Townsend SW, Rasmussen M, Clutton-Brock T, Manser MB (2012) Flexible alarm calling in meerkats: the role of the social environment and predation urgency. Behav Ecol 23:1360–1364
Trivers RL (1972) Mother-offspring conflict. Am Zool 12:648–648
Tyack P (1981) Interactions between singing Hawaiian humpback whales and conspecifics nearby. Behav Ecol Sociobiol 8:105–116
Tyack PL (1983) Differential response of humpback whales, Megaptera novaeangliae, to playback of song or social sounds. Behav Ecol Sociobiol 13:49–55
Tyack P, Whitehead H (1983) Male competition in large groups of wintering humpback whales. Behaviour 83:132–154
Ung D, Amy M, Leboucher G (2011) Heaven it's my wife! Male canaries conceal extra-pair courtships but increase aggressions when their mate watches. PLoS One 6:e22686
Waas JR (1988) Acoustic displays facilitate courtship in little blue penguins, Eudyptula minor. Anim Behav 36:366–371
Wade MJ, Shuster SM, Demuth JP (2003) Sexual selection favors female-biased sex ratios: the balance between the opposing forces of sex-ratio selection and sexual selection. Am Nat 162:403–414
Ward S, Speakman JR, Slater PJB (2003) The energy cost of song in the canary, Serinus canaria. Anim Behav 66:893–902
Winn HE, Winn LK (1978) Song of humpback whale Megaptera novaeangliae in West Indies. Mar Biol 47:97–114
Zajonc RB (1965) Social facilitation. Science 149:269–274
Zuberbühler K (2008) Audience effects. Curr Biol 18:R189–R190
Acknowledgments
The authors would like to thank everyone involved in the Humpback Acoustic Research Collaboration (HARC), and BRAHSS (the Behavioral Response of Australian Humpback whales to Seismic Surveys), in particular the numerous volunteers who donated their time and energy to this project. We also thank David Paton for his invaluable field expertise and Eric Kniest for his continued support in the development of Cyclopes and later VADAR. The authors would particularly like to acknowledge Dr. Douglas Cato for his continued advice and guidance and Jennifer Stollery for consolidating much of the raw data used for this study. Finally, the authors would like to thank the two anonymous reviewers for the valuable input.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by The US Office of Naval Research (2002–2004), the Australian Defence Science and Technology Organisation (2002–2008), the Australian Marine Mammal Centre division of the Australian Antarctic Division (2008) and (together) the Joint Industry Programme on E&P Sound and Marine Life, and the United States Bureau of Ocean Energy Management (BOEM) (2010 and 2011).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All work was carried out under permits from the Australian government Department of Environment and Water Resources and the Queensland Environmental Protection Agency and with animal ethics approval from the University of Queensland.
Informed consent
Informed consent was not required.
Additional information
Communicated by S. D. Twiss
Rights and permissions
About this article
Cite this article
Dunlop, R.A., Noad, M.J. The “risky” business of singing: tactical use of song during joining by male humpback whales. Behav Ecol Sociobiol 70, 2149–2160 (2016). https://doi.org/10.1007/s00265-016-2218-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2218-8