Abstract
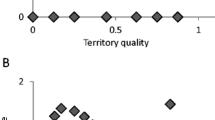
Models of sexual selection predict that socially monogamous females may gain direct or indirect (genetic) benefits by mating with multiple males. We addressed current hypotheses by investigating how, in the socially monogamous blue-black grassquit (Volatinia jacarina), male courtship and territory quality varied with social and extrapair paternity. Males of this tropical granivorous passerine exhibit multimodal displays integrating motor (leap displays) and acoustic components. Across 3 years, we found that extrapair paternity ranged from 8 to 34 % of all nestlings and from 11 to 47 % of all broods. Extrapair and socially paired male territories had similar seed densities. Females preferred to pair socially with males executing higher leaps, but no other male display characteristic associated with paternity loss and extrapair fertilizations. Extrapair and social mates did not differ in genetic similarity to female partners nor in inbreeding levels. Additionally, inbreeding and body condition of extrapair and within-pair nestlings did not differ. Thus, not only did we reject the direct benefits hypothesis for extrapair copulations, but our results also did not support the additive and nonadditive genetic benefits hypotheses. Instead, we found support for benefits through selection of potentially “good fathers,” specifically for females that chose to pair socially with males exhibiting enhanced performance in their displays.
Significance statement
Multiple mating by females is intriguing because resulting advantages seem improbable. However, access to resources, genetic compatibility with the sexual partner and good gene transmission to the offspring are possible explanations for this behavior in several animals, including socially monogamous species. We investigated potential benefits in a socially monogamous neotropical bird, the blue-black grassquit. Males attract females using a sexual display of repeated leap flights synchronized with a song. We found that when selecting social mates, females favor higher-leaping males, an attribute associated with enhanced body condition that could indicate the capacity for better parenting and also be inherited by the offspring. Yet, when choosing extrapair males, females did not appear to base choices on leap parameters, vocal attributes, and genetic compatibility. These results do not suggest benefits for multiple mating by females, but show that selection of males in good physical condition can influence choice for social mates.

Similar content being viewed by others
References
Aguilar TM, Maia R, Santos ESA, Macedo RH (2008) Parasite levels in blue-black grassquits correlate with male displays but not female mate preference. Behav Ecol 19:292–301
Akçay E, Roughgarden J (2007) Extra-pair paternity in birds: review of the genetic benefits. Evol Ecol Res 9:855–868
Alberto F (2009) MsatAllele_1.0: an R package to visualize the binning of microsatellite alleles. J Hered 100:394–397
Alderton CC (1963) The breeding behavior of the blue-black grassquit. Condor 65:154–162
Almeida JB, Macedo RH (2001) Lek-like mating system of the monogamous blue-black grassquit. Auk 118:404–411
Amos W, Wilmer JW, Fullard K, Burg TM, Croxall JP, Bloch D, Coulson T (2001) The influence of parental relatedness on reproductive success. Proc R Soc Lond B 268:2021–2027
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, New Jersey
Andersson M, Simmons LW (2006) Sexual selection and mate choice. Trends Ecol Evol 21:296–302
Aparicio JM, Ortego J, Cordero PJ (2006) What should we weigh to estimate heterozygosity, alleles or loci? Mol Ecol 15:4659–4665
Arct A, Drobniak SM, Cicho M (2015) Genetic similarity between mates predicts extrapair paternity—a meta-analysis of bird studies. Behav Ecol 26:959–968
Arnqvist G, Kirkpatrick M (2005) The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am Nat 165:S26–S37
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Barske J, Schlinger BA, Wikelski M, Fusani L (2011) Female choice for male motor skills. Proc R Soc Lond B 278:3523–3528
Bateman AJ (1948) Intra-sexual selection in Drosophila. Heredity 2:349–368
Bradbury JW, Vehrencamp SL (1998) Principles of animal communication, 2nd edn. Sinauer Associates, Sunderland, MA
Brooks RC, Griffith SC (2010) Mate choice. In: Westneat DF, Fox CW (eds) Evolutionary behavioral ecology. Oxford University Press, Oxford, pp. 416–433
Brown JL (1997) A theory of mate choice based on heterozygosity. Behav Ecol 8:60–65
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York, NY
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35
Byers J, Waits L (2006) Good genes sexual selection in nature. P Natl Acad Sci USA 103:16343–16345
Byers J, Hebets EA, Podos J (2010) Female mate choice based upon male motor performance. Anim Behav 79:771–778
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev 78:575–595
Carvalho CBV, Macedo RH, Graves JA (2006) Breeding strategies of a socially monogamous neotropical passerine: extra-pair fertilizations, behavior, and morphology. Condor 108:579–590
Catchpole CK, Slater PJB (2008) Bird song: biological themes and variations, 2nd edn. Cambridge University Press, Cambridge
Cockburn A, Osmond HL, Mulder RA, Green DJ, Double MC (2003) Divorce, dispersal and incest avoidance in the cooperatively breeding superb fairy-wren Malurus cyaneus. J Anim Ecol 72:189–202
Cohas A, Bonenfant C, Gaillard J-M, Allainé D (2007) Are extra-pair young better than within-pair young? A comparison of survival and dominance in alpine marmot. J Anim Ecol 76:771–781
Cooper BG, Goller F (2004) Multimodal signals: enhancement and constraint of song motor patterns by visual display. Science 303:544–546
Costa FJV, Macedo RH (2005) Coccidian oocyst parasitism in the blue-black grassquit: influence on secondary sex ornaments and body condition. Anim Behav 70:1401–1409
Darwin C (1871) The descent of man, and selection in relation to sex. John Murray, London, UK
Dias A (2009) Comparação e descrição de parâmetros acústicos do canto de Volatinia jacarina (Aves: Emberizidae) no contexto da seleção sexual. Dissertation, Universidade de Brasília
Dias RI, Macedo RH (2011) Nest predation versus resources in a Neotropical passerine: constraints of the food limitation hypothesis. Ornis Fenn 88:30–39
Dias RI, Castilho L, Macedo RH (2010) Experimental evidence that sexual displays are costly for nest survival. Ethology 116:1011–1019
Diniz P, Ramos DM, Macedo RH (2015) Attractive males are less than adequate dads in a multimodal signalling passerine. Anim Behav 102:109–117
Doucet SM (2002) Structural plumage coloration, male body size, and condition in the blue-black grassquit. Condor 104:30–38
DuVal EH (2007) Cooperative display and lekking behavior of the lance-tailed manakin (Chiroxiphia lanceolata). Auk 124:1168–1185
Elias DO, Lee N, Hebets EA, Mason AC (2006) Seismic signal production in a wolf spider: parallel versus serial multi-component signals. J Exp Biol 209:1074–1084
Fandiño-Mariño H, Vielliard JME (2004) Complex communication signals: the case of the blue-black grassquit Volatinia jacarina (Aves, Emberizidae) song. Part I-A structural analysis. An Acad Bras Cienc 76:325–334
Foerster K, Delhey K, Johnsen A, Lifjeld JT, Kempenaers B (2003) Females increase offspring heterozygosity and fitness through extra-pair matings. Nature 425:714–717
Forsman AM, Vogel LA, Sakaluk SK, Johnson BG, Masters BS, Johnson SL, Thompson CF (2008) Female house wrens (Troglodytes aedon) increase the size, but not immunocompetence, of their offspring through extra-pair mating. Mol Ecol 17:3697–3706
Forstmeier W, Nakagawa S, Griffith SC, Kempenaers B (2014) Female extra-pair mating: adaptation or genetic constraint? Trends Ecol Evol 29:456–464
Freeman-Gallant CR, Taff CC, Morin DF, Dunn PO, Whittingham LA, Tsang SM (2009) Sexual selection, multiple male ornaments, and age- and condition-dependent signaling in the common yellowthroat. Evolution 64:1007–1017
Gerlach NM, McGlothlin JW, Parker PG, Ketterson ED (2011) Promiscuous mating produces offspring with higher lifetime fitness. Proc R Soc Lond B 279:860–866
Gowaty PA (1996) Battles of the sexes and origins of monogamy. In: Black JM (ed) Partnerships in birds: the study of monogamy. Oxford University Press, London, pp. 21–52
Gray EM (1997) Female red-winged blackbirds accrue material benefits from copulating with extra-pair males. Anim Behav 53:625–639
Gray B, Bailey NW, Poon M, Zuk M (2014) Multimodal signal compensation: do field crickets shift sexual signal modality after the loss of acoustic communication? Anim Behav 93:243–248
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212
Grunst AS, Grunst ML (2014) Multiple sexual pigments, assortative social pairing, and genetic paternity in the yellow warbler (Setophaga petechia). Behav Ecol Sociobiol:1451–1463
Hardy OJ, Vekemans X (2002) SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620
Hebets EA, Papaj DR (2005) Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57:197–214
Hill CE, Akçay Ç, Campbell SE, Beecher MD (2011) Extrapair paternity, song, and genetic quality in song sparrows. Behav Ecol 22:73–81
Hoffman JI, Forcada J, Trathan PN, Amos W (2007) Female fur seals show active choice for males that are heterozygous and unrelated. Nature 445:912–914
Hsu Y-H, Schroeder J, Winney I, Burke T, Nakagawa S (2014) Costly infidelity: low lifetime fitness of extra-pair offspring in a passerine bird. Evolution, 2873–2884
Hsu Y-H, Schroeder J, Winney I, Burke T, Nakagawa S (2015) Are extra-pair males different from cuckolded males? A case study and a meta-analytic examination. Mol Ecol 24:1558–1571
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64
Johnstone RA (1996) Multiple displays in animal communication: “backup signals” and “multiple messages.”. Philos T Roy Soc B 351:329–338
Johnstone RA (1997) The evolution of animal signals. In: Krebs JR, Davies NB (eds) Behavioural ecology, 4th edn. Blackwell Publishing, Oxford, pp. 155–178
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Kokko H, Jennions MD, Brooks R (2006) Unifying and testing models of sexual selection. Annu Rev Ecol Evol S 37:43–66
Lehtonen TK, Kvarnemo C (2015) Odour cues from suitors’ nests determine mating success in a fish. Biol Lett 11:20150021
Lukianchuk KC, Doucet SM (2014) Cooperative courtship display in Long-tailed Manakins Chiroxiphia linearis: predictors of courtship success revealed through full characterization of display. J Ornithol 155:729–743
Maia R, Macedo RH (2011) Achieving luster: prenuptial molt pattern predicts iridescent structural coloration in blue-black grassquits. J Ornithol 152:243–252
Manica LT, Podos J, Graves J, Macedo RH (2013) Flights of fancy: mating behavior, displays and ornamentation in a neotropical bird. In: Macedo R, Machado G (eds) Sexual selection. Perspectives and models from the Neotropics. Academic Press, San Diego, CA, pp. 391–407
Manica LT, Maia R, Dias A, Podos J, Macedo RH (2014) Vocal output predicts territory quality in a Neotropical songbird. Behav Process 109:21–26
Manica LT, Macedo RH, Graves J, Podos J (2016) Vigor and skill in the acrobatic mating display of a neotropical songbird. Behav Ecol (in press)
Mazerolle MJ (2010) AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 1.25, https://www.r-project.org
Michalczyk Ł, Millard AL, Martin OY, Lumley AJ, Emerson BC, Chapman T, Gage MJG (2011) Inbreeding promotes female promiscuity. Science 333:1739–1742
Møller AP, Pomiankowski A (1993) Why have birds got multiple sexual ornaments? Behav Ecol Sociobiol 32:167–176
Møller AP, Saino N, Taramino G, Galeotti P, Ferrario S (1998) Paternity and multiple signaling: effects of a secondary sexual character and song on paternity in the barn swallow. Am Nat 151:236–242
Neff BD, Pitcher TE (2005) Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol Ecol 14:19–38
Nichols HJ, Cant MA, Sanderson JL (2015) Adjustment of costly extra-group paternity according to inbreeding risk in a cooperative mammal. Behav Ecol 26:1486–1494
Nowicki S, Peters S, Podos J (1998) Song learning, early nutrition, and sexual selection in songbirds. Am Zool 38:179–190
O’Loghlen AL, Rothstein SI (2010a) Multimodal signalling in a songbird: male audiovisual displays vary significantly by social context in brown-headed cowbirds. Anim Behav 79:1285–1292
O’Loghlen AL, Rothstein SI (2010b) It’s not just the song: male visual displays enhance female sexual responses to song in brown-headed cowbirds. Condor 112:615–621
Partan S, Marler P (2005) Issues in the classification of multimodal communication signals. Am Nat 166:231–245
Podos J (1997) A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae). Evolution 51:537–551
Queller D, Goodnight K (1989) Estimating relatedness using genetic markers. Evolution 43:258–275
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/
Reid JM, Arcese P, Sardell RJ, Keller LF (2011) Additive genetic variance, heritability, and inbreeding depression in male extra-pair reproductive success. Am Nat 177:177–187
Reid JM, Arcese P, Keller LF, Germain RR, Duthie AB, Losdat S, Wolak ME, Nietlisbach P (2015) Quantifying inbreeding avoidance through extra-pair reproduction. Evolution 69:59–74
Rousset F (2008) GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour 8:103–106
Rubenstein DR (2007) Female extrapair mate choice in a cooperative breeder: trading sex for help and increasing offspring heterozygosity. Proc R Soc Lond B 274:1895–1903
Schmoll T, Schurr FM, Winkel W, Epplen JT, Lubjuhn T (2009) Lifespan, lifetime reproductive performance and paternity loss of within-pair and extra-pair offspring in the coal tit Periparus ater. Proc R Soc Lond B 276:337–345
Schneider C, Rasband W, Eliceiri K (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Searcy WA, Nowicki S (2005) The evolution of animal communication: reliability and deception in signaling systems. Princeton University Press, Princeton, NJ
Seutin G, White BN, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analyses. Can J Zool 69:82–90
Sick H (2001) Ornitologia brasileira. Editora Nova Fronteira, Rio de Janeiro, RJ
Simmons LW (2005) The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu Rev Ecol Evol S 36:125–146
Taff CC, Steinberger D, Clark C, Belinsky K, Sacks H, Freeman-Gallant CR, Dunn PO, Whittingham LA (2012) Multimodal sexual selection in a warbler: plumage and song are related to different fitness components. Anim Behav 84:813–821
Tarvin KA, Webster MS, Tuttle EM, Pruett-Jones S (2005) Genetic similarity of social mates predicts the level of extrapair paternity in splendid fairy-wrens. Anim Behav 70:945–955
Tryjanowski P, Hromada M (2005) Do males of the great grey shrike, Lanius excubitor, trade food for extrapair copulations? Anim Behav 69:529–533
van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Varian-Ramos CW, Webster MS (2012) Extrapair copulations reduce inbreeding for female red-backed fairy-wrens, Malurus melanocephalus. Anim Behav 83:857–864
Wagner RH (1998) Hidden leks: sexual selection and the clustering of avian territories. In: Parker P, Burley N (eds) Avian reproductive tactics: female and male perspectives. Ornithological Monographs. Allen Press, Lawrence, KS, pp. 123–145
Westneat DF, Stewart IRK (2003) Extra-pair paternity in birds: causes, correlates, and conflict. Annu Rev Ecol Evol S 34:365–396
Whittingham LA, Dunn PO (2010) Fitness benefits of polyandry for experienced females. Mol Ecol 19:2328–2335
Winternitz JC, Promerova M, Polakova R, Vinker M, Schnitzer J, Munclinger P, Babik W, Radwan J, Bryja J, Albrecht T (2015) Effects of heterozygosity and MHC diversity on patterns of extra-pair paternity in the socially monogamous scarlet rosefinch. Behav Ecol Sociobiol 69:459–469
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Method Ecol Evol 1:13–14
Acknowledgments
We thank all assistants that contributed to both fieldwork and video data processing, Tanya Sneddon for assistance in the Molecular Ecology Laboratory at University of St Andrews and two anonymous reviewers for suggestions that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (AEX 4837/14-2), the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (471945/2013-7, GM/GD 142255/2012-2), the National Science Foundation (IOS-1028964), the Student Research Grant of the Animal Behavior Society, the University of St Andrews (UMGM7014), and the Universidade de Brasília. Logistic support was provided by Universidade de Brasília, University of Massachusetts - Amherst, and University of St. Andrews.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All methods used in this study are in accordance with ethical standards and Brazilian laws and were approved by the relevant authorities: Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis – IBAMA (license no. 17765-1) and by the Centro Nacional de Pesquisas para Conservação das Aves Silvestres – CEMAVE (license no. 1301).
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Communicated by C. M. Garcia
Electronic supplementary material
Online Resource 1
This video shows a male blue-black grassquit executing leap displays. (M4 V 8860 kb)
Online Resource 2
This video shows two examples of male blue-black grassquit leap displays: a higher and a lower leap display. (M4 V 16229 kb)
Online Resource 3
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Manica, L.T., Graves, J.A., Podos, J. et al. Multimodal flight display of a neotropical songbird predicts social pairing but not extrapair mating success. Behav Ecol Sociobiol 70, 2039–2052 (2016). https://doi.org/10.1007/s00265-016-2208-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2208-x