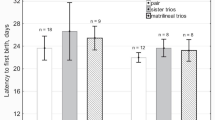
Abstract
In mammals, social tolerance among females, the philopatric sex, is formed through continued physical proximity between kin after offspring are weaned. However, the benefits of continued close association may be outweighed by costs such as local resource competition and risk of inbreeding. We hypothesized that for ‘philopatric females’, a flexible tendency towards either natal dispersal or philopatry is an important behavioral response to changes in social conditions. We examined this using an asocial rodent, Apodemus speciosus, which exhibits two discrete breeding seasons, one in spring and the second in autumn. Daughters and mothers were shown to recognize each other as kin at the time of weaning in both seasons. In spring, some mothers reproduced twice, and some first-litter daughters matured and reproduced in the same season. In autumn, however, only mothers reproduced, and there were no second litters. In spring, the proportion of natal dispersers was higher among weaned offspring whose mother remained present than those whose mother was absent, while in autumn, natal dispersal was more frequent when the mother was absent than when she remained. Sons dispersed earlier than their female littermates. Population density alone is insufficient to explain these patterns. We suggest that variable levels of reproductive competition between female kin result in seasonal differences in female natal dispersal. Breeding condition can be modulated by environmental factors, and the promotion of reproductive activity of females in spring may cause natal dispersal of daughters, while the inhibition of reproductive activity in autumn may permit philopatry.



Similar content being viewed by others
References
Adamczewska KA (1961) Intensity of reproduction of the Apodemus flavicollis (Melchior, 1843) during the period of 1954–1959. Acta Theriol 5:1–21
Anderson DR, Burnham KP, Thompson WL (2000) Null hypothesis testing: problems, prevalence, and an alternative. J Wildl Manag 64:912–923
Arnaud CM, Dobson FS, Murie JO (2012) Philopatry and within-colony movements in Columbian ground squirrels. Mol Ecol 21:493–504
Beery AK, Routman DM, Zucker I (2009) Same-sex social behavior in meadow voles: multiple and rapid formation of attachments. Physiol Behav 97:52–57
Bronson FH (1985) Mammalian reproduction: an ecological perspective. Biol Reprod 32:1–26
Bronson FH (2009) Climate change and seasonal reproduction in mammals. Phil Trans R Soc B 364:3331–3340
Broström G, Holmberg H (2011) glmmML: generalized linear models with clustered data: fixed and random effects models. Comput Stat Data Anal 55:3123–3134
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Clarke AL, Sæther BE, Røskaft E (1997) Sex biases in avian dispersal: a reappraisal. Oikos 79:429–438
Clobert J, De Fraipont M, Danchin E (2008) Evolution of dispersal. In: Danchin E, Giraldeu LA, Cezilly F (eds) Behavioural ecology. Oxford University Press, Oxford, pp 323–335
Clobert J, Ims RA, Rousset F (2004) Causes, mechanisms and consequences of dispersal. In: Hanski I, Gaggiotti OE (eds) Ecology, genetics, and evolution of metapopulations. Academic, Amsterdam, pp 307–335
Clutton-Brock TH, Lukas D (2012) The evolution of social philopatry and dispersal in female mammals. Mol Ecol 21:472–492
Corbet GB (1978) Mammals of the Palaearctic region: a taxonomic review. British Museum (Natural History) and Cornell University Press, London
Cote J, Clobert J (2007) Social personalities influence natal dispersal in a lizard. Proc R Soc Lond B 274:383–390
Dingemanse NJ, Both C, van Noordwijk AJ, Rutten AL, Drent PJ (2003) Natal dispersal and personalities in great tits (Parus major). Proc R Soc B 270:741–747
Dobson FS (1982) Competition for mates and predominant juvenile male dispersal in mammals. Anim Behav 30:1183–1192
Edelman AJ, Koprowski JL (2007) Communal nesting in asocial Abert’s squirrels: the role of social thermoregulation and breeding strategy. Ethology 113:147–154
Eto T, Sakamoto SH, Okubo Y, Koshimoto C, Kashimura A, Morita T (2014) Huddling facilitates expression of daily torpor in the large Japanese field mouse Apodemus speciosus. Physiol Behav 133:22–29
Gilbert C, McCafferty D, Le Maho Y, Martrette JM, Giroud S, Blanc S, Ancel A (2010) One for all and all for one: the energetic benefits of huddling in endotherms. Biol Rev 85:545–569
Goldman BD (2003) Pattern of melatonin secretion mediates transfer of photoperiod information from mother to fetus in mammals. Sci Signal 192:pe29
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Hanski I, Selonen V (2009) Female-biased natal dispersal in the Siberian flying squirrel. Behav Ecol 20:60–67
Harold JG (1982) Kin recognition in white-footed deermice (Peromyscus leucopus). Anim Behav 30:497–505
Holmes WG (1986) Kin recognition by phenotype matching in female Belding’s ground squirrels. Anim Behav 34:38–47
Ims RA (1990) Determinants of natal dispersal and space use in grey-sided voles, Clethrionomys rufocanus: a combined field and laboratory experiment. Oikos 57:106–113
Jacquot JJ, Vessey SH (1995) Influence of the natal environment on dispersal of white-footed mice. Behav Ecol Sociobiol 37:407–412
Jans JE, Leon M (1983) The effects of lactation and ambient temperature on the body temperature of female Norway rats. Physiol Behav 30:959–961
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948
Kokko H, Lopez-Sepulcre A, Morrell LJ (2006) From hawks and doves to self-consistent games of territorial behaviour. Am Nat 167:901–912
Lacey EA, Sherman PW (2007) The ecology of sociality in rodents. In: Wolff JO, Sherman PW (eds) Rodent societies: an ecological and evolutionary perspective. University of Chicago Press, Chicago, pp 243–254
Lambin X (1994) Sex ratio variation in relation to female philopatry in Townsend’s voles. J Anim Ecol 63:945–953
Lambin X, Aars J, Piertney SB (2001) Dispersal, intraspecific competition, kin competition and kin facilitation: a review of the empirical evidence. In: Clobert J, Danchin E, Dhondt AA, Nichols JD (eds) Dispersal. Oxford University Press, Oxford, pp 110–122
Le Galliard JF, Ferriere R, Dieckmann U (2005) Adaptive evolution of social traits: origin, trajectories, and correlations of altruism and mobility. Am Nat 165:206–224
Le Galliard JF, Gundersen G, Andreassen HP, Stenseth NC (2006) Natal dispersal, interactions among siblings and intrasexual competition. Behav Ecol 17:733–740
Le Galliard JF, Gundersen G, Steen H (2007) Mother–offspring interactions do not affect natal dispersal in a small rodent. Behav Ecol 18:665–673
Le Galliard JF, Remy A, Ims RF, Lambin X (2012) Patterns and processes of dispersal behaviour in arvicoline rodents. Mol Ecol 21:505–523
Legan SJ, Karsch FJ (1980) Photoperiodic control of seasonal breeding in ewes: modulation of the negative feedback action of estradiol. Biol Reprod 23:1061–1068
Lott DF (1991) Intraspecific variation in the social systems of wild vertebrates. Cambridge University Press, London
Maloiy GMO, Rugangazi BM, Clemens ET (1988) Physiology of the dik-dik antelope. Comp Biochem Physiol A Physiol 91:1–8
Miller TE, Shaw AK, Inouye BD, Neubert MG (2011) Sex-biased dispersal and the speed of two-sex invasions. Am Nat 177:549–561
Nakata K, Saitoh T, Iwasa MA (2009) Apodemus speciosus, Apodemus argenteus. In: Ohdachi SD, Ishibashi Y, Iwasa MA, Saitoh T (eds) The wild mammals of Japan. Shoukadoh Book Sellers and the Mammalogical Society of Japan, Kyoto, pp 169–173
Oh HS, Mōri T (1998) Growth, development and reproduction in captive of the large Japanese field mouse, Apodemus speciosus (Rodentia, Muridae). J Fac Agr Kyushu Univ 43:397–408
Oka T (1992) Home range and mating system of two sympatric field mouse species, Apodemus speciosus and Apodemus argenteus. Ecol Res 7:163–169
Pinheiro J, Bates D, DebRoy S, Sarkar D (2012) nlme: linear and nonlinear mixed effects models. R package version 3.1–103, http://CRAN.R-project.org/package=nlme
Porter RH, Tepper VJ, White DM (1981) Experiential influences on the development of huddling preferences and “sibling” recognition in spiny mice. Dev Psychol 14:375–382
Prychodko W (1958) Effect of aggregation of laboratory mice (Mus musculus) on food intake at different temperatures. Ecology 39:500–503
Quirici V, Faugeron S, Hayes LD, Ebensperger LA (2011) The influence of group size on natal dispersal in the communally rearing and semifossorial rodent, Octodon degus. Behav Ecol Sociobiol 65:787–798
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org
Sakamoto SH, Suzuki SN, Degawa Y, Koshimoto C, Suzuki RO (2012) Seasonal habitat partitioning between sympatric terrestrial and semi-arboreal Japanese wood mice, Apodemus speciosus and A. argenteus in spatially heterogeneous environment. Mamm Stud 37:261–272
Schoepf I, Schradin C (2012) Better off alone! Reproductive competition and ecological constraints determine sociality in the African striped mouse (Rhabdomys pumilio). J Anim Ecol 81:649–656
Schradin C, König B, Pillay N (2010) Reproductive competition favours solitary living while ecological constraints impose group-living in African striped mice. J Anim Ecol 79:515–521
Schradin C, Lindholm AK, Johannesen J, Schoepf I, Yuen CH, Köenig B, Pillay N (2012) Social flexibility and social evolution in mammals: a case study of the African striped mouse (Rhabdomys pumilio). Mol Ecol 21:541–553
Sekijima T (1995) Metabolic rates of two congeneric woodmice, Apodemus argenteus and A. speciosus (Rodentia: Muridae), in Japan. J Mamm Soc Japan 20:143–149
Selonen V, Hanski IK (2010) Movements of dispersing flying squirrels in relation to siblings and parents. Behav Ecol Sociobiol 64:1019–1027
Selonen V, Hanski IK, Wistbacka R (2014) Communal nesting is explained by subsequent mating rather than kinship or thermoregulation in the Siberian flying squirrel. Behav Ecol Sociobiol 68:971–980
Shields WM (1987) Dispersal and mating systems: investigating their causal connections. In: Chepko-Sade BD, Halpin ZT (eds) Mammalian dispersal patterns: the effects of social structure on population genetics. University of Chicago Press, Chicago, pp 3–24
Shintaku Y, Kageyama M, Motokawa M (2010) Differential growth patterns in two seasonal cohorts of the large Japanese field mouse Apodemus speciosus. J Mammal 91:1168–1177
Sinervo B, Clobert J (2003) Morphs, dispersal behavior, genetic similarity, and the evolution of cooperation. Science 300:1949–1951
Stamps JA (2001) Habitat selection by dispersers: integrating proximate and ultimate approaches. In: Clobert J, Danchin E, Dhondt AA, Nichols JD (eds) Dispersal. Oxford University Press, Oxford, pp 230–242
Stenseth NC, Lidicker WZ Jr (1992) The study of dispersal: a conceptual guide. In: Stenseth NC, Lidicker WZ Jr (eds) Animal dispersal. Springer, Berlin, pp 5–20
Therneau T (2011) coxme: mixed effects Cox models. R package version 2.1–3, http://CRAN.R-project.org/package=coxme
van Aarde RJ (1985) Reproduction in captive female Cape porcupines (Hystrix africaeaustralis). J Reprod Fertil 75:577–582
Waldman B (1988) The ecology of kin recognition. Annu Rev Ecol Syst 19:543–571
Waser PM (1985) Does competition drive dispersal? Ecology 66:1170–1175
Williams CT, Gorrell JC, Lane JE, McAdam AG, Humphries MM, Boutin S (2013) Communal nesting in an ‘asocial’ mammal: social thermoregulation among spatially dispersed kin. Behav Ecol Sociobiol 67:757–763
Wolff JO (1992) Parents suppress reproduction and stimulate dispersal in opposite-sex juvenile white-footed mice. Nature 359:409–410
Acknowledgments
We are grateful to Takashi Saitou, Tadashi Suzuki, Tamotsu Kusano, and Fumio Hayashi for their advice on this study. We would like to thank Yusuke Sakai and Ryousuke Ozaki for help with animal maintenance. We thank the Agricultural and Forestry Research Center of the University of Tsukuba for permission to conduct the field study within the site. This study was supported by Grant-in-Aid for Challenging Exploratory Research from JSPS (Grant Numbers 24657018 to Shinsuke H. Sakamoto; 23650236 to Chihiro Koshimoto) and by grants from the University of Miyazaki (Support Program for Integrated Research Project for Human and Veterinary Medicine, and Athena research fellowships). This study was presented at the workshop “Dispersal male and Philopatric female” at the annual meeting of the Mammalogical Society, Japan, in 2012. We thank Takuya Shimada, Naoki Onishi, Yamato Tsuji, and Akihiro Yamane, the organizers of the workshop. We are grateful to the two reviewers who provided insightful comments on an earlier version of the text.
Ethical standards
The field study was conducted in accordance with Japanese animal welfare legislation, and the experimental procedures were examined and approved by the Animal Experimentation Committee of the University of Miyazaki (2010–516, 2012–002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Korpimäki
Rights and permissions
About this article
Cite this article
Sakamoto, S.H., Eto, T., Okubo, Y. et al. The effects of maternal presence on natal dispersal are seasonally flexible in an asocial rodent. Behav Ecol Sociobiol 69, 1075–1084 (2015). https://doi.org/10.1007/s00265-015-1920-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1920-2