Abstract
Spreading reproduction across time or space can optimize fitness by minimizing the risks for offspring survival in varying and unpredictable environments. Poison frogs (Dendrobatidae) are characterized by complex spatial and reproductive behaviour, such as territoriality, prolonged courtship and parental care. The partitioning of larvae from terrestrial clutches across several water bodies is mainly known from species with carnivorous tadpoles that allocate their tadpoles in very small pools, where limited food availability is accompanied by an increased risk of cannibalism. However, little is known about the deposition behaviour of non-carnivorous species that use medium-sized to large pools. In the present study, we investigated whether the Neotropical poison frog Allobates femoralis exhibits brood-partitioning behaviour when males transport tadpoles 3 weeks after oviposition. We sampled 30 artificial water bodies for tadpoles, which we genotyped at seven highly polymorphic microsatellite loci. Based on the reconstructed pedigree, we show that A. femoralis males distribute larvae of single and of successive clutches across several water bodies. The number of pools used was significantly associated with the number of clutches per male. Ninety-three percent of the males that were assigned to more than one clutch spread their tadpoles across several water bodies. Given the highly variable and unpredictable biotic and abiotic conditions in tropical rainforest, at the spatial scale of the study species’ behaviour, we interpret this behaviour as bet-hedging to improve offspring survival.
Similar content being viewed by others
Introduction
Variable and unpredictable environmental conditions are expected to favour the evolution of bet-hedging strategies (Beaumont et al. 2009; Simons 2011). By spreading the risk of mortality across time and/or space, individuals can optimize fitness by reducing the variance in reproductive success in favour of long-term risk reduction (Hopper 1999; Crean and Marshall 2009; Olofsson et al. 2009; Simons 2011). Several studies have demonstrated fitness benefits of diversified reproductive strategies; e.g. allocating reproduction across several mating partners (Fox and Rauter 2003; Mäkinen et al. 2007; Sarhan and Kokko 2007; Garcia-Gonzalez et al. 2015), distributing eggs across several sites (Root and Kareiva 1984; Byrne et al. 2009; Andersson and Åhlund 2012) and variably initiating egg incubation in order to promote hatching asynchrony (Laaksonen 2004). However, compared to the comprehensive theoretical literature on bet-hedging strategies, empirical datasets from natural populations, particularly on parental behaviours after hatching, are rather scarce.
Among anurans, parental care is mainly found in terrestrial breeders in the humid tropics (Wells 2007). Terrestrial oviposition has evolved independently several times, presumably as an adaptation to high aquatic predation pressure on eggs (Magnusson and Hero 1991) and the risk of reduced fertilization success due to stray sperm from rivals in aquatic environments (Roberts and Byrne 2011). The tadpoles of most terrestrial breeders still complete their development until metamorphosis in water, a dilemma which some species solve by transporting their tadpoles to aquatic sites (Wells 2007).
Pre-metamorphic mortality in amphibians is around 90 % in most species (Vonesh and De la Cruz 2002), caused by abiotic and biotic factors, such as predation (Magnusson and Hero 1991), pond desiccation (Richter-Boix et al. 2011), inter- and intra-specific competition for food (Gonzalez et al. 2011) as well as parasitism and pathogen infections (Kriger and Hero 2007; Rhoden and Bolek 2011). As the choice of high-quality larval developmental sites has profound effects on offspring survival and thus on the parent’s reproductive success, selection should therefore drive the evolution of parental behaviours that assess the quality of breeding sites in order to maximize offspring growth and survival. Such discriminatory behaviour regarding larval deposition sites has been shown in several anuran species (Spieler and Linsenmair 1997; Summers 1999; Summers and McKeon 2004; Schulte et al. 2011). However, natural environments of amphibian larvae are also characterized by highly unpredictable variability (e.g. weather, predators, etc.), making it almost impossible for the transporting parent to reliably predict the future quality of a pool for the entire period of larval development. Depositing offspring (of single and/or successive clutches) in several pools therefore would constitute a suitable bet-hedging strategy to minimize the risk of total offspring loss in face of environmental uncertainty (cf. Byrne et al. 2009).
Neotropical poison frogs (Dendrobatidae) show complex behaviours such as territoriality, elaborate courtship and parental care. All species deposit their eggs outside of water, and after hatching, most of them transport the tadpoles to water bodies, where they complete their larval development until metamorphosis (Lötters et al. 2007). In some dendrobatids, adults select deposition sites according to food availability (Poelman et al. 2013) or the presence of predators or conspecifics (Brown et al. 2008; Schulte et al. 2011; McKeon and Summers 2013; Rojas 2014). Species that have carnivorous tadpoles which they transport to small pools, such as bromeliads or small tree holes, are known to distribute their larvae across several sites (Caldwell and de Araújo 1998; Summers 1999). Limited food availability accompanied by an increased risk of cannibalism presumably selected for this behaviour (Summers and Amos 1997; Brown et al. 2008; see also Stynoski et al. 2014). Little is known about the deposition behaviour of non-carnivorous species that use medium-sized pools, where nutrients are usually not limited.
Given the highly unpredictable biotic and abiotic impacts in temporal aquatic environments, we expect that brood-partitioning has evolved also in species that use medium-sized pools. We tested this hypothesis in the Neotropical poison frog Allobates femoralis by using field observations and microsatellite genotypes of adults and tadpoles from one reproductive season in order to identify whether males distribute their tadpoles from successive and/or single clutches across several water bodies.
Methods
Study species
Males of the Neotropical frog A. femoralis (Boulenger 1884; Anura: Dendrobatidae) are highly territorial and announce territory possession by a prominent advertisement call (Narins et al. 2003; Ringler et al. 2011). Females are inter-dispersed between male territories and show non-aggressive site fidelity (Ringler et al. 2009, 2012). Pair formation, courtship and mating occur in male territories (Roithmair 1992; Montanarin et al. 2011). Both sexes are highly polygynandrous throughout the reproductive season. Females can produce one clutch every 8 days on average (Weygoldt 1980, in captivity) and males were observed to guard up to five clutches simultaneously (Ursprung et al. 2011). Tadpole transport by male A. femoralis takes place 15–20 days after oviposition, which carry on average 8 (range = 1–25) tadpoles to small to medium-sized terrestrial pools (e.g. medium-sized temporal pools, floodplains, peccary wallows, footprints, palm fronds, holes in fallen trees), usually located outside a male’s territory (Ringler et al. 2013). In captivity, A. femoralis distribute their tadpoles when provided with several water pools, and tadpoles require 40–50 days of aquatic development until metamorphosis (Weygoldt 1980).
Our study population of A. femoralis is located in a lowland rainforest near the field camp ‘Saut Pararè’ (4° 02′ N, 52° 41′ W) in the nature reserve ‘Les Nourages’, French Guiana (details in Ursprung et al. 2011; Ringler et al. 2014). Thirty artificial pools (30 × 30 × 20 cm) were placed in a 6 by 5 array with ∼10 m distance between pools in the centre of our study plot in 2009, originally for another study on the effects of reproductive resource supplementation in A. femoralis (Ringler et al. 2015). Each pool was filled with approximately 500 cm3 of leaf litter to match natural forest floor cover and filled with rainwater.
Sampling and genotyping
Data on the spatial locations of all adult individuals as well as microsatellite genotypes were already available from a previous study (Ringler et al. 2013). We sampled the artificial pools in January and April 2010 to capture tadpole-transport events over the entire study period and to avoid sampling larvae from one clutch repeatedly. We randomly sampled approximately one third of all tadpoles per pool and sacrificed them in 96 % ethanol. Additionally, we assessed the presence of potential predators of A. femoralis at the first sampling event (i.e. we noted all potential predators of A. femoralis tadpoles that were present at the respective pool). DNA extraction, genotyping and parentage assignments followed the protocol of Ursprung et al. (2011). We used all markers that were available for the Guiana population when samples were genetically analysed.
Brood-partitioning behaviour
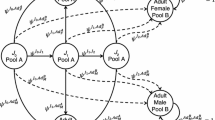
We reconstructed patterns of tadpole deposition by A. femoralis males via parentage assignments of larval and adult genotypes. Specifically, we aimed to reveal whether males distribute their larvae of single and/or successive clutches across multiple pools. We only included tadpoles with at least five unambiguously genotyped loci. Given that females produce multiple clutches throughout the breeding season, but rarely mate with the same male twice (Ursprung et al. 2011; Ringler et al. 2012), and males only transport tadpoles from a single clutch at a time (Ringler et al. 2013), we were able to distinguish between inter- and intra-clutch partitioning of A. femoralis males. We assumed that tadpole full-siblings (as inferred by the parentage analysis) from the same sampling cohort (January or April) belonged to the same clutch. Full-siblings that were sampled in different cohorts, as well as paternal half-sibs, were considered to originate from separate clutch depositions and were thus treated as distinct transportation events of the respective fathers. Given the larval development time in water until metamorphosis of about 45 days, tadpoles from the first sampling cohort would have already left the pool at the second sampling event. We only included clutches that were represented by at least two full-sibs, and for the analyses of inter-clutch partitioning, we considered only males that were assigned to at least two clutches. All statistical tests were performed in IBM SPSS Statistics 20.0.0. Normality of variables was tested with the Kolmogorov–Smirnov test. Medium values, interquartile ranges (iqr) and non-parametric tests were applied in cases where normality was rejected.
Results
We counted a total of 2595 A. femoralis tadpoles in our artificial pools across both samplings, with pools containing a median number of 19 tadpoles per pool (iqr = 7.5–67.25, max = 363, Fig. 1). Dragonfly larvae were the most common aquatic predators in our artificial pools. Pools contained also a few singular carnivorous tadpoles of Dendrobates tinctorius, which usually get deposited in elevated tree holes. We observed that the number of tadpoles per pool significantly decreased with increasing number of dragonfly larvae (n = 9, Spearman’s ρ = −0.717, y = −12.013x + 59.25, R 2 = 0.37, p = 0.03; Fig. 2). Pools without any dragonfly larvae contained up to 135 tadpoles (mean ± SD = 46.14 ± 39.38). During the study period, we sporadically observed the presence of other potential predators of A. femoralis tadpoles in and at the pools: spiders, snakes (e.g. Helicops angulatus), terrestrial crabs and twist-necked turtles (e.g. Platemys platycephala). In three cases, we observed a dramatic decrease in water quality caused by fallen fruits, dead animals and faeces (e.g. of howler monkeys) that started to ferment and rot inside the pools, which lead to a total loss of tadpoles inside the affected pools. We sampled in total 414 tadpoles by collecting up to 19 tadpoles per pool and sampling session (median = 6, iqr = 3.75–10).
We had to exclude 19 tadpoles from further analyses due to low PCR amplification success. Of the remaining 395 tadpoles, 340 could be unambiguously assigned to a known father from the 2010 population. Ninety-three (43.9 %) of the 212 A. femoralis males in our study had produced offspring. Our genetic analysis indicated that of these 93 males, 65 sired more than 2 of the analysed tadpoles and 44 males sired tadpoles from more than one clutch. Forty-one (93.18 %) of the 44 males distributed their tadpoles (from single or successive clutches) across several water bodies (Fig. 3). Seventeen males spread the larvae of single clutches over either two (n = 14) or three (n = 3) pools, respectively. Not a single male with more than three clutches carried its tadpoles to only one single pool (Fig. 4). On average, we found larvae of three males within one pool at a given sampling session (iqr = 1.75–4). Individual males who were assigned to more than one tadpole used a median of two pools (iqr = 1–3, n = 65). The number of pools used per male was significantly associated with the number of identified clutches (n = 93, r = 0.877, linear regression fit: y = 0.825x + 0.303, R 2 = 0.769, p < 0.001; Fig. 4). One additional clutch per male translated into, on average, 0.825 (standard error = 0.047) additional used pools. This relationship also remained significant when all males with only one assigned tadpole were removed from the analysis, but the effect size was then reduced to additional 0.44 (standard error = 0.064) pools, on average, per added clutch (n = 65, r = 0.843, linear regression fit: y = 0.789x + 0.444, R 2 = 0.710, p < 0.001).
Discussion
In this study, we provide evidence for brood-partitioning behaviour in A. femoralis. The number of pools where males used to deposit their tadpoles increased significantly with their number of clutches. On average, 0.82 additional pools were used per additional clutch by each male. All males with more than three clutches used multiple pools for larval deposition. This demonstrates that A. femoralis males generally distribute their offspring from successive clutches, and to a lesser extent also from single clutches, across several pools.
Based on the heterogeneity of ephemeral aquatic environments, there are presumably large differences in quality with respect to tadpole development. Small natural water bodies at our study site were observed to quickly dry out when exposed to direct sunlight in periods of little rainfall (cf. Roth and Jackson 1987; Summers 1999; pers. obs. by all authors). Large pools are less susceptible to desiccation, but in turn are associated with increased predation risk by invertebrate larvae (Alford 1999). In our experimental design, pool size was constant (approx. 20 L), because pools were originally established for a study on reproductive resource supplementation (Ringler et al. 2015). We found dragonfly larvae and to a lesser extent heterospecific carnivorous tadpoles (e.g. D. tinctorius) as common aquatic predators of A. femoralis tadpoles at our study site. Also, spiders, snakes and tortoises were identified as potential predators of A. femoralis tadpoles. Although cannibalism is common in many dendrobatids (Caldwell and de Araújo 1998; Summers and Symula 2001), A. femoralis tadpoles do not show this behaviour (Weygoldt 1980, obs. in captivity; ER pers. obs.). Consequently, male A. femoralis do not avoid placing their offspring with other conspecific tadpoles.
In pools where dragonfly larvae were present, the number of tadpoles per pool significantly decreased with increasing number of dragonfly larvae. We cannot unambiguously distinguish whether this effect was caused by prior predation by the dragonfly larvae or whether it is a result of differential deposition of fewer tadpoles into pools with many dragonfly larvae. It has been shown by McKeon and Summers (2013) that A. femoralis males try to assess the presence of predators at specific water bodies immediately before larval deposition and adjust their deposition behaviour accordingly. However, predators could also enter the pool after tadpoles have been released. Thus, even if transporting males assess predator presence prior to tadpole deposition, these males cannot be certain of a low predation risk for their brood in the near future. In addition to predation, other confounding and highly unpredictable factors were identified, such as fruits, faeces or dead animals that fell into the pools and severely degraded water quality (i.e. no surviving tadpoles were detected inside these pools). In contrast, other pools contained up to 363 tadpoles when they were sampled (Fig. 1). We conclude that there are highly diverse and unforeseeable threats regarding tadpole development across pools—a crucial prerequisite for bet-hedging strategies to evolve.
The costs of tadpole transport for the carriers, such as conspicuousness to predators (Crump 1995), energetic effort (Simon 1983), or lost mating opportunities (Townsend 1986) will increase with the time spent distributing tadpoles. We expect increased costs for both inter- and intra-clutch partitioning (e.g. increased searching effort or longer duration when allocating tadpoles of a single clutch across multiple pools) compared to males always using the same pool for larval deposition. However, the resulting increase in tadpole survival might outweigh the associated costs for the transporting individual. Our data do now allow the assessment of whether patterns of tadpole distribution ultimately influence individual reproductive success in A. femoralis. But given the high variation in quality among (natural) water bodies, the low predictability of risks for the entire period of larval development and the presumably small increase in costs for the transporting parent (compared to single-pool use), a strong selective benefit of bet-hedging strategies can be assumed (cf. Leimar 2009). The allocation of larvae across multiple sites may act as a risk-spreading strategy to overcome the risk of total offspring loss, by combining advantages from between- and within-generation bet-hedging (i.e. inter- and intra-clutch partitioning), respectively (cf. conservative bet hedging: Philippi and Seger 1989; see also Summers 1990 and Starrfelt and Kokko 2012). We hypothesize that such brood-spreading behaviour has evolved as an adaptation to environmental uncertainty.
We cannot unambiguously differentiate whether males actively approached specific pools or whether they roamed through the area in search of suitable water bodies. However, A. femoralis males can accurately navigate in familiar areas around their territories (Pašukonis et al. 2014a, b), and previous studies indicate a strategic behaviour rather than an aimless search for suitable tadpole deposition sites (Ringler et al. 2013). As naturally occurring tadpole-deposition sites are generally neither common nor evenly distributed, the use of known pools would be preferable to repeated random searching. Preliminary data on tadpole transport trajectories show straight approach trajectories (Pašukonis, unpublished data). Hence, we presume that males actively approach the pools they know to distribute their larvae.
The observed brood-partitioning behaviour might also have causes other than bet-hedging, such as reduced competition among kin or resource depletion (cf. Smith 1990; Saidapur and Girish 2001; see also Pfennig 1990). Given that A. femoralis tadpoles are herbivorous and nutrients in medium-sized pools are not a limiting factor (Brown et al. 2008 and references therein), we expect that the negative impact of tadpole density is rather negligible. The variation in the number of tadpoles across pools was high (Fig. 1); thus, it is highly unlikely that the observed brood-spreading strategy has evolved as a mechanism to avoid high larval density in water bodies. However, the observed brood-partitioning behaviour is not necessarily driven by one single mechanism. Beside the benefits of bet-hedging, other positive effects due to allocating kin across space might have helped to shift the cost-benefit ratio in favour of a brood-spreading strategy.
In dendrobatid frogs, we find the evolutionary transition from species carrying many tadpoles (e.g. whole clutches) into large water bodies to species that allocate single tadpoles each to distinct small-sized pools, such as bromeliad axils or other phytotelmata (Weygoldt 1987; Brown et al. 2010). We hypothesize that the group-wise partitioning of tadpoles, particularly of single clutches, in species that use medium-sized pools might have been a transitional evolutionary step towards the deposition of only single tadpoles in very small pools (cf. Summers and McKeon 2004). While large bodies of water are generally associated with high predation risk, small pools offer almost predator-free environments (see Brown et al. 2008 and references therein). At the same time, food availability for larvae decreases with decreasing size of the water body. Consequentially, species with carnivorous tadpoles allocate their larvae singly into small phytotelmata to avoid larval cannibalism among siblings (Summers 1999; Summers and McKeon 2004) and regularly return to the deposition site in order to provision their young with unfertilized eggs (Poelman and Dicke 2007; Brown et al. 2009). However, these modalities are only the two extremes of a continuum, with many dendrobatid species actually using medium-sized pools for larval deposition (Brown et al. 2010). In our study, we show that brood-partitioning behaviour can occur in a species that uses medium-sized water bodies. This finding demonstrates that brood-spreading behaviour can evolve also in the absence of cannibalism and fully independent from post-deposition provisioning of aquatic larvae.
References
Alford RA (1999) Ecology: resource use, competition and predation. In: McDiarmid RW, Altig R (eds) Tadpoles. The biology of anuran larvae. University of Chicago Press, Chicago, pp 240–278
Andersson M, Åhlund M (2012) Don’t put all your eggs in one nest: spread them and cut time at risk. Am Nat 180:354–363
Beaumont HJ, Gallie J, Kost C, Ferguson GC, Rainey PB (2009) Experimental evolution of bet hedging. Nature 462:90–93
Boulenger GA (1884) On a collection of frogs from Yurimaguas, Huallaga River, Northern Peru. Proc Zool Soc London 1883:635–638
Brown JL, Twomey E, Morales V, Summers K (2008) Phytotelm size in relation to parental care and mating strategies in two species of Peruvian poison frogs. Behaviour 145:1139–1165
Brown JL, Morales V, Summers K (2009) Tactical reproductive parasitism via larval cannibalism in Peruvian poison frogs. Biol Lett 5:148–151
Brown JL, Morales V, Summers K (2010) A key ecological trait drove the evolution of biparental care and monogamy in an amphibian. Am Nat 175:436–446
Byrne PG, Keogh SJ, Keogh JS (2009) Extreme sequential polyandry insures against nest failure in a frog. Proc R Soc Lond B 276:115–120
Caldwell JP, de Araújo MC (1998) Cannibalistic interactions resulting from indiscriminate predatory behavior in tadpoles of poison frogs (Anura: Dendrobatidae). Biotropica 30:92–103
Crean AJ, Marshall DJ (2009) Coping with environmental uncertainty: dynamic bet hedging as a maternal effect. Philos Trans R Soc B 364:1087–1096
Crump ML (1995) Parental care. In: Sullivan BK, Heatwole H (eds) Social behaviour. Surrey Beatty, Chipping Norton, pp 518–567
Fox CW, Rauter CM (2003) Bet-hedging and the evolution of multiple mating. Evol Ecol Res 5:273–286
Garcia-Gonzalez F, Yasui Y, Evans JP (2015) Mating portfolios: bet-hedging, sexual selection and female multiple mating. Proc R Soc Lond B 282:20141525
Gonzalez SC, Touchon JC, Vonesh JR (2011) Interactions between competition and predation shape early growth and survival of two Neotropical hylid tadpoles. Biotropica 43:633–639
Hopper KR (1999) Risk-spreading and bet-hedging in insect population biology. Annu Rev Entomol 44:535–560
Kriger KM, Hero J (2007) The chytrid fungus Batrachochytrium dendrobatidis is non-randomly distributed across amphibian breeding habitats. Divers Distrib 13:781–788
Laaksonen T (2004) Hatching asynchrony as a bet-hedging strategy—an offspring diversity hypothesis. Oikos 104:616–620
Leimar O (2009) Environmental and genetic cues in the evolution of phenotypic polymorphism. Evol Ecol 23:125–135
Lötters S, Jungfer K, Henkel FW, Schmidt W (2007) Poison frogs. Biology, species and captive husbandry. Edition Chimaira, Frankfurt am Main, Germany
Magnusson WE, Hero J (1991) Predation and the evolution of complex oviposition behaviour in Amazon rainforest frogs. Oecologia 86:310–318
Mäkinen T, Panova M, André C (2007) High levels of multiple paternity in Littorina saxatilis: hedging the bets? J Hered 98:705–711
McKeon CS, Summers K (2013) Predator driven reproductive behavior in a tropical frog. Evol Ecol 27:725–737
Montanarin A, Kaefer IL, Lima Pimentel A (2011) Courtship and mating behaviour of the brilliant-thighed frog Allobates femoralis from Central Amazonia: implications for the study of a species complex. Ethol Ecol Evol 23:141–150
Narins PM, Hödl W, Grabul DS (2003) Bimodal signal requisite for agonistic behavior in a dart-poison frog, Epipedobates femoralis. Proc Natl Acad Sci U S A 100:577–580
Olofsson H, Ripa J, Jonzén N (2009) Bet-hedging as an evolutionary game: the trade-off between egg size and number. Proc R Soc Lond B 276:2963–2969
Pašukonis A, Loretto M, Landler L, Ringler M, Hödl W (2014a) Homing trajectories and initial orientation in a Neotropical territorial frog, Allobates femoralis (Dendrobatidae). Front Zool 11:29
Pašukonis A, Warrington I, Ringler M, Hödl W (2014b) Poison frogs rely on experience to find the way home in the rainforest. Biol Lett 10:20140642
Pfennig DW (1990) The adaptive significance of an environmentally-cued developmental switch in an anuran tadpole. Oecologia 85:101–107
Philippi T, Seger J (1989) Hedging one’s evolutionary bets, revisited. Trends Ecol Evol 4:41–44
Poelman EH, Dicke M (2007) Offering offspring as food to cannibals: oviposition strategies of Amazonian poison frogs (Dendrobates ventrimaculatus). Evol Ecol 21:215–227
Poelman EH, Wijngaarden RAP, Raaijmakers CE (2013) Amazon poison frogs (Ranitomeya amazonica) use different phytotelm characteristics to determine their suitability for egg and tadpole deposition. Evol Ecol 27:661–674
Rhoden HR, Bolek MG (2011) Distribution and reproductive strategies of Gyrinicola batrachiensis (Oxyuroidea: Pharyngodonidae) in larvae of eight species of amphibians from Nebraska. J Parasitol 97:629–635
Richter-Boix A, Tejedo M, Rezende EL (2011) Evolution and plasticity of anuran larval development in response to desiccation. A comparative analysis. Ecol Evol 1:15–25
Ringler M, Ursprung E, Hödl W (2009) Site fidelity and patterns of short- and long-term movement in the brilliant-thighed poison frog Allobates femoralis (Aromobatidae). Behav Ecol Sociobiol 63:1281–1293
Ringler M, Ringler E, Magaña Mendoza D, Hödl W (2011) Intrusion experiments to measure territory size: development of the method, tests through simulations, and application in the frog Allobates femoralis. PLoS ONE 6:e25844
Ringler E, Ringler M, Jehle R, Hödl W (2012) The female perspective of mating in A. femoralis, a territorial frog with paternal care—a spatial and genetic analysis. PLoS ONE 7:e40237
Ringler E, Pašukonis A, Hödl W, Ringler M (2013) Tadpole transport logistics in a Neotropical poison frog: indications for strategic planning and adaptive plasticity in anuran parental care. Front Zool 10:1–10
Ringler M, Mangione R, Pašukonis A et al (2014) High-resolution forest mapping for behavioural studies in the Nature Reserve ‘Les Nouragues’, French Guiana. J Maps. doi:10.1080/17445647.2014.972995
Ringler M, Hödl W, Ringler E (2015) Populations, pools, and peccaries: simulating the impact of ecosystem engineers on rainforest frogs. Behav Ecol. doi:10.1093/beheco/aru243
Roberts JD, Byrne PG (2011) Polyandry, sperm competition, and the evolution of anuran amphibians. Adv Stud Behav 43:1–53
Roithmair ME (1992) Territoriality and male mating success in the dart-poison frog, Epipedobates femoralis (Dendrobatidae, Anura). Ethology 92:331–343
Rojas B (2014) Strange parental decisions: fathers of the dyeing poison frog deposit their tadpoles in pools occupied by large cannibals. Behav Ecol Sociobiol 68:551–559
Root RB, Kareiva PM (1984) The search for resources by cabbage butterflies (Pieris rapae): ecological consequences and adaptive significance of Markovian movements in a patchy environment. Ecology 65:147–165
Roth AH, Jackson JF (1987) The effect of pool size on recruitment of predatory insects and on mortality in a larval anuran. Herpetologica 43:224–232
Saidapur SK, Girish S (2001) Growth and metamorphosis of Bufo melanostictus tadpoles: effects of kinship and density. J Herpetol 35:249–254
Sarhan A, Kokko H (2007) Multiple mating in the Glanville fritillary butterfly: a case of within-generation bet hedging? Evolution 61:606–616
Schulte LM, Yeager J, Schulte R, Veith M, Werner P, Beck LA, Lötters S (2011) The smell of success: choice of larval rearing sites by means of chemical cues in a Peruvian poison frog. Anim Behav 81:1147–1154
Simon MP (1983) The ecology of parental care in a terrestrial breeding frog from New Guinea. Behav Ecol Sociobiol 14:61–67
Simons AM (2011) Modes of response to environmental change and the elusive empirical evidence for bet hedging. Proc R Soc Lond B 278:1601–1609
Smith DC (1990) Population structure and competition among kin in the chorus frog (Pseudacris triseriata). Evolution 44:1529–1541
Spieler M, Linsenmair KE (1997) Choice of optimal oviposition sites by Hoplobatrachus occipitalis (Anura: Ranidae) in an unpredictable and patchy environment. Oecologia 109:184–199
Starrfelt J, Kokko H (2012) Bet-hedging—a triple trade-off between means, variances and correlations. Biol Rev 87:742–755
Stynoski JL, Shelton G, Stynoski P (2014) Maternally derived chemical defenses are an effective deterrent against some predators of poison frog tadpoles (Oophaga pumilio). Biol Lett 10:20140187
Summers K (1990) Paternal care and the cost of polygyny in the green dart-poison frog. Behav Ecol Sociobiol 27:307–313
Summers K (1999) The effects of cannibalism on Amazonian poison frog egg and tadpole deposition and survivorship in Heliconia axil pools. Oecologia 119:557–564
Summers K, Amos W (1997) Behavioral, ecological, and molecular genetic analyses of reproductive strategies in the Amazonian dart-poison frog, Dendrobates ventrimaculatus. Behav Ecol 8:260–267
Summers K, McKeon CS (2004) The evolutionary ecology of phytotelmata use in Neotropical poison frogs. Misc Publ Mus Zool Univ Michigan 193:55–73
Summers K, Symula R (2001) Cannibalism and kin discrimination in tadpoles of the Amazonian poison frog, Dendrobates ventrimaculatus, in the field. Herpetol J 11:17–21
Townsend DS (1986) The costs of male parental care and its evolution in a neotropical frog. Behav Ecol Sociobiol 19:187–195
Ursprung E, Ringler M, Jehle R, Hödl W (2011) Strong male/male competition allows for nonchoosy females: high levels of polygynandry in a territorial frog with paternal care. Mol Ecol 20:1759–1771
Vonesh JR, De la Cruz O (2002) Complex life cycles and density dependence: assessing the contribution of egg mortality to amphibian declines. Oecologia 133:325–333
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Weygoldt P (1980) Zur Fortpflanzungsbiologie von Phyllobates femoralis (Boulenger) im Terrarium. Salamandra 16:215–226
Weygoldt P (1987) Evolution of parental care in dart poison frogs (Amphibia: Anura: Dendrobatidae). Z Zool Syst Evol 25:51–67
Acknowledgments
The work in the field and laboratory was funded by the University of Vienna (‘Förderstipendium’), the Centre national de la recherche scientifique (CNRS, ‘Nouragues Grant’, PI: MR) and by the Austrian Science Fund (FWF: P18811, PI: WH; P24788-B22, PI: ER). The Nouragues Ecological Research Station is supported by the CNRS. All necessary permissions were acquired from the CNRS and the DEAL Guyane. We are grateful to Alexandra Fischer-Pardow, Florian Ulm, Johannes Döhlemann and Mathias Fernandez for the assistance with tadpole sampling, to Matthias Nemeth and Norbert Milasowszky for the statistical advice and to Gesche Westphal-Fitch for the language editing. Barbara Fischer and two anonymous reviewers provided valuable comments on the manuscript.
Ethical standards
We confirm that there are no competing interests for any of the authors. All research presented in the manuscript was conducted in accordance with all applicable laws and rules set forth by their governments and institutions, and all necessary permits were in hand when the research was conducted.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Summers
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Erich, M., Ringler, M., Hödl, W. et al. Brood-partitioning behaviour in unpredictable environments: hedging the bets?. Behav Ecol Sociobiol 69, 1011–1017 (2015). https://doi.org/10.1007/s00265-015-1913-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1913-1