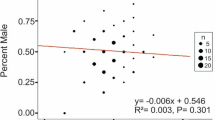
Abstract
Offspring sex ratios in mammals vary in potentially adaptive yet unpredictable ways. An integrative approach that simultaneously examines proximate and ultimate explanations of mammalian sex ratios would greatly advance the field. We examined the importance of maternal glucose and stress hormones for offspring sex (male or female) as mechanisms associated with the Trivers–Willard and the local resource competition hypotheses of sex allocation. We tested this framework in a marsupial mammal, the tammar wallaby (Macropus eugenii). Mothers that were better able to maintain body condition over the driest part of the year, a presumptive proxy for local resource availability, were more likely to produce daughters (the philopatric sex), consistent with local resource competition. Maternal glucose was correlated with offspring sex, but in the opposite direction than we predicted—higher maternal glucose was associated with female pouch young. These patterns, however, were not consistent across the 2 years of our study. Maternal stress hormone metabolites measured from fecal samples did not predict glucose or offspring sex. A causative glucose mechanism may underlie an adaptive strategy for mothers with high local resources (high glucose) to produce philopatric daughters that will benefit from inheriting resource access. Examining species-specific relationships between glucose and offspring sex across mammals could provide crucial insight into the disparate ecological and selective pressures faced by mammals with respect to offspring sex ratio.


Similar content being viewed by others
References
Banks SC, Knight EJ, Dubach JE, Lindenmayer DB (2008) Microhabitat heterogeneity influences offspring sex allocation and spatial kin structure in possums. J Animal Ecol 77:1250–1256
Barker JM (1961) Metabolism of carbohydrate and volatile fatty acids in marsupial, Setonix brachyurus. Q J Exp Physiol CMS 46:54–68
Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A (2010) Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc Natl Acad Sci U S A 107:3394–3399
Blumstein D, Evans CS, Daniel JC (1999) An experimental study of behavioural group size effects in tammar wallabies, Macropus eugenii. Anim Behav 58:351–360
Blumstein DT, Ardron JG, Evans CS (2002a) Kin discrimination in a macropod marsupial. Ethology 108:815–823
Blumstein DT, Daniel JC, Ardron JG, Evans CS (2002b) Does feeding competition influence tammar wallaby time allocation? Ethology 108:937–945
Blumstein DT, Daniel JC, Springett BP (2004) A test of the multi-predator hypothesis: rapid loss of antipredator behavior after 130 years of isolation. Ethology 110:919–934
Bonier F, Martin PR, Wingfield JC (2007) Maternal corticosteroids influence primary offspring sex ratio in a free-ranging passerine bird. Behav Ecol 18:1045–1050
Cameron EZ (2004) Facultative adjustment of mammalian sex ratios in support of the Trivers-Willard hypothesis: evidence for a mechanism. Proc R Soc Lond B 271:1723–1728
Cameron EZ, Lemons PR, Bateman PW, Bennett NC (2008) Experimental alteration of litter sex ratios in a mammal. Proc R Soc Lond B 275:323–327
Catling PC, Vinson GP (1976) Adrenocortical hormones in the neonate and pouch young of the tammar wallaby, Macropus eugenii. J Endocrinol 69:447–448
Chambers BK (2009) Human disturbance affects the ecology and population dynamics of the tammar wallaby, Macropus eugenii, on Garden Island. Dissertation, University of Western Australia, Western Australia
Chambers BK, Bencini R (2010) Impact of human disturbance on the population dynamics and ecology of tammar wallabies on Garden Island, Western Australia. In: Coulson G, Eldridge M (eds) Macropods: the biology of kangaroos, wallabies and rat-kangaroos. CSIRO, Collingwood, pp 211–218
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Clark AB (1978) Sex ratio and local resource competition in a prosimian primate. Science 201:163–165
Cockburn A (1994) Adaptive sex allocation by brood reduction in Antechinus. Behav Ecol Sociobiol 35:53–62
Cockburn A, Scott MP, Dickman CR (1985) Sex ratio and intrasexual kin competition in mammals. Oecologia 66:427–429
Cooley H, Janssens PA (1977) Metabolic effects of infusion of cortisol and adrenocorticotrophin in the tammar wallaby (Macropus eugenii Desmarest). Gen Comp Endocrinol 33:352–358
Cork SJ, Dove H (1989) Lactation in the tammar wallaby (Macropus eugenii). 2. Intake of milk components and maternal allocation of energy. J Zool 219:399–409
Creel S, Dantzer B, Goymann W, Rubenstein DR (2013) The ecology of stress: effects of the social environment. Funct Ecol 27:66–80
Croft DB (1989) Social organization of the Macropodoidea. In: Grigg G, Jarman P, Hume I (eds) Kangaroos, wallabies and rat-kangaroos. Surrey Beatty & Sons Pty Limited, Chipping Norton, pp 505–525
Davison MJ, Ward SJ (1998) Prenatal bias in sex ratios in a marsupial, Antechinus agilis. Proc R Soc Lond B 265:2095–2099
Ewen KR, Templesmith PD, Bowden DL, Marinopoulos J, Renfree MB, Yan H (1993) DNA-fingerprinting in relation to male-dominance and paternity in a captive colony of tammar wallabies (Macropus eugenii). J Reprod Fert 99:33–37
Gam AE, Mendonça MT, Navara KJ (2011) Acute corticosterone treatment prior to ovulation biases offspring sex ratios towards males in zebra finches Taeniopygia guttata. J Avian Biol 42:253–258
Gardner DK, Larman MG, Thouas GA (2010) Sex-related physiology of the preimplantation embryo. Mol Hum Reprod 16:539–547
Goltsman M, Kruchenkova EP, Sergeev S, Johnson PJ, Macdonald DW (2005) Effects of food availability on dispersal and cub sex ratio in the Mednyi Arctic fox. Behav Ecol Sociobiol 59:198–206
Green B, Merchant JC, Newgrain K (1988) Milk consumption and energetics of growth in pouch young of the tammar wallaby, Macropus eugenii. Aust J Zool 36:217–227
Gutierrez-Adan A, Granados J, Pintado B, de la Fuente J (2001) Influence of glucose on the sex ratio of bovine IVM/IVF embryos cultured in vitro. Reprod Fert Develop 13:361–365
Helle S, Laaksonen T, Adamsson A, Paranko J, Huitu O (2008) Female field voles with high testosterone and glucose levels produce male-biased litters. Anim Behav 75:1031–1039
Hewison AJM, Gaillard J-M (1999) Successful sons or advantaged daughters? The Trivers–Willard model and sex-biased maternal investment in ungulates. Trends Ecol Evol 14:229–234
Hiraiwa-Hasegawa M (1993) Skewed sex ratios in primates: should high-ranking mothers have daughters or sons? Trends Ecol Evol 8:395–400
Hume ID (1982) Digestive physiology and nutrition of marsupials. Cambridge University Press, Cambridge
Hynes EF (2005) Mating sequence, dominance and paternity success in captive male tammar wallabies. Reproduction 130:123–130
Inns RW (1980) Ecology of the Kangaroo Island wallaby, Macropus eugenii (Desmarest) in Flinders Chase National Park. Dissertation, University of Adelaide, Kangaroo Island
Isaac JL, Krockenberger AK, Johnson CN (2005) Adaptive sex allocation in relation to life-history in the Common brushtail possum, Trichosurus vulpecula. J Anim Ecol 74:552–558
Janssens PA, Hinds LA (1981) Long-term effects of corticosteroid administration in the tammar wallaby, Macropus eugenii. Gen Comp Endocrinol 45:56–60
Johnson CN (1988) Dispersal and the sex ratio at birth in primates. Nature 332:726–728
Johnson CN (1989) Dispersal and philopatry in the Macropodoids. In: Grigg G, Jarman P, Hume I (eds) Kangaroos, wallabies and rat-kangaroos. Surrey Beatty & Sons Pty Limited, Chipping Norton, pp 593–602
Johnson CN, Ritchie EG (2002) Adaptive biases in offspring sex ratios established before birth in a marsupial, the Common brushtail possum Trichosurus vulpecula. Behav Ecol 13:653–656
Johnson CN, Clinchy M, Taylor AC, Krebs CJ, Jarman PJ, Payne A, Ritchie EG (2001) Adjustment of offspring sex ratios in relation to the availability of resources for philopatric offspring in the Common brushtail possum. Proc R Soc Lond B 268:2001–2005
Julliard R (2000) Sex-specific dispersal in spatially varying environments leads to habitat-dependent evolutionary stable offspring sex ratios. Behavioral Ecology 11:421–428
Kruuk LE, Clutton-Brock TH, Albon SD, Pemberton JM, Guinness FE (1999) Population density affects sex ratio variation in red deer. Nature 399:459–461
Larson MA, Kimura K, Kubisch HM, Roberts RM (2001) Sexual dimorphism among bovine embryos in their ability to make the transition to expanded blastocyst and in the expression of the signaling molecule IFN-tau. Proc Natl Acad Sci U S A 98:9677–9682
Love OP, Chin EH, Wynne‐Edwards KE, Williams TD (2005) Stress hormones: a link between maternal condition and sex‐biased reproductive investment. Am Nat 166:751–766
MacDonald AJ, FitzSimmons NN, Chambers B, Renfree MB, Sarre SD (2013) Sex-linked and autosomal microsatellites provide new insights into island populations of the tammar wallaby. Heredity 2013:1–10
Martin JGA, Festa-Bianchet M (2011) Sex ratio bias and reproductive strategies: what sex to produce when? Ecology 92:441–449
McKenzie S, Deane EM (2003) The effects of age, season, and gender on serum cortisol levels in the tammar wallaby, Macropus eugenii. Gen Comp Endocrinol 133:273–278
McKenzie S, Deane EM (2005) Faecal corticosteroid levels as an indicator of well-being in the tammar wallaby, Macropus Eugenii. Comp Biochem Phys A 140:81–87
McKenzie S, Deane EM, Burnett L (2004) Are serum cortisol levels a reliable indicator of wellbeing in the tammar wallaby, Macropus eugenii? Comp Biochem Phys A 138:341–348
Miller EJ, Eldridge MBD, Herbert CA (2010) Dominance and paternity in the tammar wallaby. In: Coulson G, Eldridge M (eds) Macropods: the biology of kangaroos, wallabies and rat-kangaroos. CSIRO, Collingwood, pp 77–86
Pelletier F, Réale D, Garant D, Coltman DW, Festa-Bianchet M (2007) Selection on heritable seasonal phenotypic plasticity of body mass. Evolution 61:1969–1979
Pike TW, Petrie M (2005) Maternal body condition and plasma hormones affect offspring sex ratio in peafowl. Anim Behav 70:745–751
Pike TW, Petrie M (2006) Experimental evidence that corticosterone affects offspring sex ratios in quail. Proc R Soc Lond B 273:1093–1098
Poole WE, Simms NG, Wood JT, Luboloa M (1991) Tables for age determination of the Kangaroo Island tammar wallaby (Macropus eugenii) from body measurements. Technical Memorandum no. 32. CSIRO, Canberra
Pryke SR, Rollins LA, Buttemer WA, Griffith SC (2011) Maternal stress to partner quality is linked to adaptive offspring sex ratio adjustment. Behav Ecol 22:717–722
Robert KA, Braun S (2012) Milk composition during lactation suggests a mechanism for male biased allocation of maternal resources in the tammar wallaby (Macropus eugenii). PLoS ONE 7:e51099
Robert KA, Schwanz LE (2011) Emerging sex allocation research in mammals: marsupials and the pouch advantage. Mammal Rev 41:1–22
Robert KA, Schwanz LE (2013) Monitoring the health status of free-ranging tammar wallabies using haematology, serum biochemistry and parasite load. J Wildlife Manag 77:1232–1243
Robert KA, Schwanz LE, Mills HR (2009) Offspring sex varies with maternal investment ability: empirical demonstration based on cross-fostering. Biol Lett 6:242–245
Ryan CP, Anderson WG, Gardiner LE, Hare JF (2012) Stress-induced sex ratios in ground squirrels: support for a mechanistic hypothesis. Behav Ecol 23:160–167
Schwanz LE, Robert KA (2012) Reproductive ecology of wild tammar wallabies in natural and developed habitats on Garden Island, Western Australia. Aust J Zool 60:111–119
Schwanz LE, Bragg JG, Charnov EL (2006) Maternal condition and facultative sex ratios in populations with overlapping generations. Am Nat 168:521–530
Sheldon BC, West SA (2004) Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am Nat 163:40–54
Silk JB (1983) Local resource competition and facultative adjustment of sex ratios in relation to competitive abilities. Am Nat 121:56–66
Simpson MJA, Simpson AE (1982) Birth sex ratios and social rank in rhesus monkey mothers. Nature 300:440–441
Spindler RE, Renfree MB, Shaw G, Gardner DK (1998) Reactivating tammar wallaby blastocysts oxidize glucose. Biol Reprod 58:1425–1431
Sunnucks P, Taylor AC (1997) Sex of pouch young related to maternal weight in Macropus eugenii and M. parma (Marsupialia: Macropodidae). Aust J Zool 45:573–578
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
Tyndale-Biscoe H, Renfree MB (1987) Reproductive physiology of marsupials. Cambridge University Press, Cambridge
Warner DA, Radder RS, Shine R (2009) Corticosterone exposure during embryonic development affects offspring growth and sex ratios in opposing directions in two lizard species with environmental sex determination. Physiol Biochem Zool 82:363–371
Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL (2000) A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120:260–275
West SA (2009) Sex allocation. Princeton University Press, Princeton
Wild G (2006) Sex ratios when helpers stay at the nest. Evolution 60:2012–2022
Wild G, West SA (2007) A sex allocation theory for vertebrates: combining local resource competition and condition‐dependent allocation. Am Nat 170:E112–E128
Williamson P, Fletcher TP, Renfree MB (1990) Testicular development and maturation of the hypothalamic-pituitary-testicular axis in the male tammar, Macropus eugenii. Reproduction 88:549–557
Acknowledgments
This research was conducted with the permission of the Australian Department of Defence. We thank J. Wann and T. Smith for assistance in accessing the animals and research facilities, and B. Chambers and R. Bencini for advice on research design, logistics, loaned traps, and lodging. Numerous field assistants helped with the trapping. Comments from C. Johnson, M. Jennions, and D. Reznick improved the manuscript. We thank E. Berkeley, F. Krikowa, B. Maher, R. McCuaig, S. Thomas, and the University of Canberra Faculty of Applied Science Molecular Lab for equipment, space, advice, and assistance with the hormonal analyses. The research was funded by a U.S. National Science Foundation International Research Fellowship (LES), a University of Western Australia Postdoctoral Research Fellowship (KAR), University of Western Australia Research Grants Scheme (KAR), and the Australian Department of Defence (KAR and LES).
Ethical standards
This research was conducted in compliance with ethical standards in Australia, under the ethical approval of the University of Western Australia’s Animal Ethics Committee (approval: RA/3/100/897) and Department of Environment and Conservation Research approval (permits: SF007185 and SF007651).
Conflict of interest
The authors have no conflict of interest regarding the publication of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. I. Schulte-Hostedde
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 35 kb)
Rights and permissions
About this article
Cite this article
Schwanz, L.E., Robert, K.A. Proximate and ultimate explanations of mammalian sex allocation in a marsupial model. Behav Ecol Sociobiol 68, 1085–1096 (2014). https://doi.org/10.1007/s00265-014-1720-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1720-0