Abstract
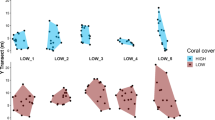
The persistence of behavioral types in situ and the drivers of persistence are central to predicting the ecological effects of intraspecific behavioral variation. We surveyed individual refuge use of mud crabs (Panopeus herbstii), a behavior related to the strength of a trait-mediated trophic cascade in oyster reefs, in the absence and presence of toadfish (Opsanus tau) predation threat. We then released these crabs into the field and using mark-recapture, measured the repeatability of this behavior in the absence and presence of threat, and how behavioral change was affected by time in the field (a month on average, up to 81 days), crab size, and sex. Because crabs exhibited some evidence of a circatidal rhythm in refuge use, we also tested how tidal height during observation influenced behavioral change. Predation threat increased refuge use, and small crabs used the refuge more than large crabs, particularly under threat. In recaptured crabs, refuge use was more repeatable under threat. Neither time in the field, crab size, crab sex, nor tidal height had any effect on behavioral change. Our results support the non-mutually exclusive hypotheses that (1) prey organisms in the presence, rather than absence, of predation threat should exhibit less behavioral variability because the fear of dying (a severe fitness consequence) should take precedence over less immediately important influences on behavior (e.g., hunger) and that (2) individual behaviors tied to fixed traits (e.g., the body size dependence of refuge use under threat in this study), rather than variable traits, should be more repeatable over time.


Similar content being viewed by others
References
Archard G, Braithwaite V (2010) The importance of wild populations in studies of animal temperament. J Zool 281:149–160
Barnwell FH (1966) Daily and tidal patterns of activity in individual fiddler crab (Genus Uca) from the Woods Hole region. Biol Bull 130:1–17
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783
Biro PA (2012) Do rapid assays predict repeatability in labile (behavioural) traits? Anim Behav 83:1295–1300
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bolnick DI, Svanback R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Bolnick DI, Amarasekare P, Araujo MS, Burger R, Levine JM, Novak M, Rudolf VHW, Schreiber SJ, Urban MC, Vasseur DA (2011) Why intraspecific trait variation matters in community ecology. Trends Ecol Evol 26:183–192
Briffa M, Greenway J (2011) High in situ repeatability of behaviour indicates animal personality in the beadlet anemone Actinia equina (Cnidaria). Plos One 6:e21963
Brown C, Braithwaite VA (2004) Size matters: a test of boldness in eight populations of the poeciliid Brachyraphis episcopi. Anim Behav 68:1325–1329
Butler S, Whittingham M, Quinn J, Cresswell W (2006) Time in captivity, individual differences and foraging behaviour in wild-caught chaffinches. Behaviour 143:535–548
Dame R, Chrzanowski T, Bildstein K, Kjerfve B, McKellar H, Nelson D, Spurrier J, Stancyk S, Stevenson H, Vernberg F, Zingmark R (1986) The outwelling hypothesis and North Inlet, South Carolina. Mar Ecol Prog Ser 33:217–229
Dingemanse NJ, Réale D (2005) Natural selection and animal personality. Behaviour 142:1165–1190
Dingemanse NJ, Both C, Drent PJ, Van Oers K, Van Noordwijk AJ (2002) Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim Behav 64:929–938
Dowling LM, Godin JGJ (2002) Refuge use in a killifish: influence of body size and nutritional state. Can J Zool 80:782–788
Dunlap JC, Loros JJ, DeCoursey PJ (2004) Chronobiology: biological timekeeping. Sinauer Associates, Sunderland
Emlen JM (1966) The role of time and energy in food preference. Am Nat 100:611–617
Ferrari C, Pasquaretta C, Carere C, Cavallone E, von Hardenberg A, Réale D (2013) Testing for the presence of coping styles in a wild mammal. Anim Behav 85:1385–1396
Fodrie FJ, Brodeur MC, Toscano BJ, Powers SP (2012) Friend or foe: conflicting demands and conditional risk taking by opportunistic scavengers. J Exp Mar Biol Ecol 422:114–121
Gabriel PO, Black JM (2010) Behavioural syndromes in Steller's jays: the role of time frames in the assessment of behavioural traits. Anim Behav 80:689–697
Gherardi F, Aquiloni L, Tricarico E (2012) Behavioral plasticity, behavioral syndromes and animal personality in crustacean decapods: an imperfect map is better than no map. Curr Zool 58:567–579
Gosling SD (2001) From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45–86
Grabowski JH (2004) Habitat complexity disrupts predator–prey interactions but not the trophic cascade on oyster reefs. Ecology 85:995–1004
Grabowski JH, Kimbro DL (2005) Predator-avoidance behavior extends trophic cascades to refuge habitats. Ecology 86:1312–1319
Griffen BD, Toscano BJ, Gatto J (2012) The role of individual behavior type in mediating indirect interactions. Ecology 93:1935–1943
Gudger EW (1910) Habits and life history of the toadfish (Opsanus tau). Bull US Bur Fish 28:1071–1109
Heinonen KB, Auster PJ (2012) Prey selection in crustacean-eating fishes following the invasion of the Asian shore crab Hemigrapsus sanguineus in a marine temperate community. J Exp Mar Biol Ecol 413:177–183
Hensley NM, Cook TC, Lang M, Petelle MB, Blumstein DT (2012) Personality and habitat segregation in giant sea anemones (Condylactis gigantea). J Exp Mar Biol Ecol 426:1–4
Herborn KA, Macleod R, Miles WT, Schofield AN, Alexander L, Arnold KE (2010) Personality in captivity reflects personality in the wild. Anim Behav 79:835–843
Hill JM (2011) Predator biomass and habitat characteristics affect the magnitude of consumptive and non-consumptive effects (NCEs): experiments between blue crabs, mud crabs, and oyster prey. Dissertation, Georgia Institute of Technology, Atlanta, GA
Hoffmann AA (2000) Laboratory and field heritabilities: some lessons from Drosophila. In: Mousseau TA, Sinervo B, Endler JA (eds) Adaptive genetic variation in the wild. Oxford University Press, New York, pp 200–218
Horvath K, Angeletti D, Nascetti G, Carere C (2013) Invertebrate welfare: an overlooked issue. Ann Ist Sup Sanità 49:9–17
Koski SE (2011) How to measure animal personality and why does it matter? integrating the psychological and biological approaches to animal personality. In: Inoue-Murayama M, Kawamura S, Weiss A (eds) From genes to animal behavior: social structures, personalities, communication by color. Springer, Tokyo, pp 115–136
Mather J (2013) The bold and the spineless: invertebrate personalities. In: Carere C, Maestripieri D (eds) Animal personalities: behavior, physiology, and evolution. The University of Chicago Press, Chicago, pp 13–35
McDonald J (1982) Divergent life history patterns in the co-occurring intertidal crabs Panopeus herbstii and Eurypanopeus depressus (Crustacea: Brachyura: Xanthidae). Mar Ecol Prog Ser 8:173–180
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956
Palmer JD (1973) Tidal rhythms: the clock control of the rhythmic physiology of marine organisms. Biol Rev 48:377–418
Pruitt JN, Ferrari MCO (2011) Intraspecific trait variants determine the nature of interspecific interactions in a habitat-forming species. Ecology 92:1902–1908
Pruitt J, Riechert S (2012) The ecological consequences of temperament in spiders. Curr Zool 58:589–596
Pruitt JN, Stachowicz JJ, Sih A (2012) Behavioral types of predator and prey jointly determine prey survival: potential implications for the maintenance of within-species behavioral variation. Am Nat 179:217–227
Réale D, Gallant BY, Leblanc M, Festa-Bianchet M (2000) Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim Behav 60:589–597
Rudin FS, Briffa M (2012) Is boldness a resource-holding potential trait? fighting prowess and changes in startle response in the sea anemone, Actinia equina. Proc R Soc B 279:1904–1910
Saigusa M (1992) Phase shift of a tidal rhythm by light–dark cycles in the semi-terrestrial crab Sesarma pictum. Biol Bull 182:257–264
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Sih A, Cote J, Evans M, Fogarty S, Pruitt J (2012) Ecological implications of behavioural syndromes. Ecol Lett 15:278–289
Stachowicz JJ, Hay M (1999) Reduced mobility is associated with compensatory feeding and increased diet breadth of marine crabs. Mar Ecol Prog Ser 188:169–178
Stamps J, Groothuis TG (2010) The development of animal personality: relevance, concepts and perspectives. Biol Rev 85:301–325
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton
Wilson ADM, Godin JGJ (2009) Boldness and behavioral syndromes in the bluegill sunfish, Lepomis macrochirus. Behav Ecol 20:231–237
Wilson CA, Dean JM, Radtke R (1982) Age, growth rate and feeding habits of the oyster toadfish, Opsanus tau (Linnaeus) in South Carolina. J Exp Mar Biol Ecol 62:251–259
Acknowledgments
Funding was provided by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-0929297 and by the National Science Foundation Grant No. OCE-1129166. We thank D.S. Wethey, J.L. Dudycha and the anonymous reviewers for the helpful discussions and suggestions that improved the manuscript.
Ethical standards
This research complies with the current laws of the United States.
Conflict of interest
We declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Breithaupt
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 177 kb)
Rights and permissions
About this article
Cite this article
Toscano, B.J., Gatto, J. & Griffen, B.D. Effect of predation threat on repeatability of individual crab behavior revealed by mark-recapture. Behav Ecol Sociobiol 68, 519–527 (2014). https://doi.org/10.1007/s00265-013-1666-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1666-7