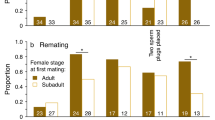
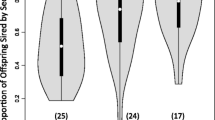
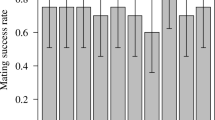
Abstract
In species without nuptial gifts or parental care, postcopulatory attendance of females by males has generally been interpreted as males guarding against sperm competition. Guarding benefits may be concurrent with attendance (the guarding-now hypothesis), or male behavior during attendance may make the female unreceptive (the guarding in absentia hypothesis). However, in addition to guarding functions, attendance may provide the male with an opportunity to influence the female's use of sperm. In haplodiploids such as hymenopterans, doing so may be beneficial because only daughters and not sons are produced sexually and so influence male reproductive success (the sex ratio hypothesis). In the parasitoid wasp Urolepis rufipes, postcopulatory attendance involved the male remaining mounted after copulation and resuming courtship. Support for the guarding-now hypothesis was limited. A male's presence on a female did not reduce the probability, or quickness, of another male mounting, and second-mounted males frequently copulated. The guarding in absentia hypothesis was not supported. Females became unreceptive soon after mating even when copulation and postcopulatory attendance were experimentally prevented. The sex ratio hypothesis was supported. Postcopulatory attendance caused females to produce more daughters. They also produced more total offspring. Thus, a male should stay and should not go even in the absence of other males, at least when opportunities for other matings are absent as in the present study. Although most studies of offspring sex ratios have focused on maternal control, this study provides an example of apparently adaptive male influence on sex ratio.




Similar content being viewed by others
References
Ablard K, Fairhurst S, Andersen G, Schaefer P, Gries G (2011) Mechanisms, functions, and fitness consequences of pre- and post-copulatory rituals of the parasitoid wasp Ooencyrtus kuvanae. Entomol Exp Appl 140:103–111. doi:10.1111/j.1570-7458.2011.01137.x
Alcock J (1994) Postinsemination associations between males and females in insects: the mate-guarding hypothesis. Ann Rev Entomol 39:1–21
Arnqvist G, Rowe L (2005) Sexual conflict. Princeton University Press, Princeton
Bloch Qazi MC, Aprille JR, Lewis SM (1998) Female role in sperm storage in the red flour beetle, Tribolium castaneum. Comp Biochem Physiol A 120:641–647
Bretman A, Gage MJG, Chapman T (2011) Quick-change artists: male plastic behavioural responses to rivals. Trends Ecol Evol 26:467–473. doi:10.1016/j.tree.2011.05.002
Brockmann HJ, Grafen A (1989) Mate conflict and male behaviour in a solitary wasp, Trypoxylon (Trypargilum) politum (Hymenoptera: Sphecidae). Anim Behav 37:232–255
Burton-Chellew MN, Koevoets T, Grillenberger BK, Sykes EM, Underwood SL, Bijlsma K, Gadau J, van de Zande L, Beukeboom LW, West SA, Shuker DM (2008) Facultative sex ratio adjustment in natural populations of wasps: cues of local mate competition and the precision of adaptation. Am Nat 172:393–404
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Cooper JL (2010) Mating behaviors, receptivity signaling and male abdomen marking of the parasitoid wasp Urolepsis rufipes. Thesis, Northern Illinois University
Cordoba-Aguilar A (2006) Sperm ejection as a possible cryptic female choice mechanism in Odonata (Insecta). Physiol Entomol 31:146–153
Crudgington HS, Siva-Jothy MT (2000) Genital damage, kicking and early death. Nature 407:855–856
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Eguchi K, Yamagishi S, Asai S, Nagata H, Hino T (2002) Helping does not enhance reproductive success of cooperatively breeding rufous vanga in Madagascar. J Anim Ecol 71:123–130. doi:10.1046/j.0021-8790.2001.00585.x
Fedina TY (2007) Cryptic female choice during spermatophore transfer in Tribolium castaneum (Coleoptera: Tenebrionidae). J Insect Physiol 53:93–98
Field SA, Keller MA (1993) Alternative mating tactics and female mimicry as post-copulatory mate-guarding behaviour in the parasitic wasp Cotesia rubecula. Anim Behav 46:1183–1189. doi:10.1006/anbe.1993.1308
Flanders SE (1939) Environmental control of sex in hymenopterous insects. Ann Entomol Soc Am 32:11–26
Flanders SE (1946) Control of sex and sex-limited polymorphism in the Hymenoptera. Q Rev Biol 21:135–143
Flanders SE (1956) The mechanisms of sex ratio regulation in the parasitic Hymenoptera. Insectes Soc 3:325–334
Grillenberger BK, Koevoets T, Burton-Chellew MN, Sykes EM, Shuker DM, Van de Zande L, Bijlsma R, Gadau J, Beukeboom LW (2008) Genetic structure of natural Nasonia vitripennis populations: validating assumptions of sex-ratio theory. Mol Ecol 17:2854–2856
Hardy ICW (1994) Sex ratio and mating structure in the parasitoid Hymenoptera. Oikos 69:3–20
Hawkes PG (1992) Sex ratio stability and male–female conflict over sex ratio control in hymenopteran parasitoids. S Afr J Sci 88:423–430
Henter HJ (2004) Constrained sex allocation in a parasitoid due to variation in male quality. J ExpBiol 17:886–896
Kajita H (1986) Role of postcopulatory courtship in insemination of two aphelinid wasps (Hymenoptera: Aphelinidae). Appl Entomol Zool 21:484–486
King BH (2010) Which sex controls the duration of postcopulatory courtship and to what effect in the parasitoid wasp Spalangia endius? Behavior 147:993–1007
King BH, Bressac C (2010) No fitness consequence of experimentally induced polyandry in a monandrous wasp. Behavior 147:85–102
King BH, Fischer CR (2005) Males mate guard in absentia through extended effects of postcopulatory courtship in the parasitoid wasp Spalangia endius. J Ins Physiol 51:1340–1345
King BH, Fischer CR (2010) Male mating history: effects on female sexual responsiveness and reproductive success in the parasitoid wasp Spalangia endius. Behav Ecol Sociobiol 64:607–615. doi:10.1007/s00265-009-0878-3
Kraaijeveld K (2009) Male genes with nowhere to hide; sexual conflict in haplodiploids. Anim Biol 59:403–415. doi:10.1163/157075509x12499949744225
Kuban KA (2012) The parasitoid wasp Urolepis rufipes: finding and choosing a mate. Thesis, Northern Illinois University
Lehner PN (1998) Handbook of ethological methods, 2nd edn. Cambridge University Press. doi:10.2277/0521637503
Leonard ML, Horn AG, Eden SF (1989) Does juvenile helping enhance breeder reproductive success? Behav Ecol Sociobiol 25:357–361. doi:10.1007/bf00302993
Linley JR, Mook MS (1975) Behavioural interaction between sexually experienced Culicoides melleus (Coquillett) (Diptera: Ceratopogonidae). Behavior 54:97–110
Matthews JR, Petersen JJ (1990) Effects of host age, host density and parent age on reproduction of the filth fly parasite Urolepis rufipes (Hymenoptera: Pteromalidae). Med Vet Entomol 4:255–260
Nichols WJ, Cossé AA, Bartelt RJ, King BH (2010) Methyl 6-methylsalicylate: a female-produced pheromone component of the parasitoid wasp Spalangia endius. J Chem Ecol 36:1140–1147
Powell JR, Graham LC, Galloway TD (2003) Development time of Urolepis rufipes (Hymenoptera: Pteromalidae) and effect of female density on offspring sex ratio and reproductive output. Proc Entomol Soc Manitoba 59:16–20
Rodriguez-Muñoz R, Bretman A, Tregenza T (2011) Guarding males protect females from predation in a wild insect. Curr Biol 21:1716–1719
Ross L, Pen I, Shuker DM (2010) Genomic conflict in scale insects: the causes and consequences of bizarre genetic systems. Biol Rev 85:807–828. doi:10.1111/j.1469-185X.2010.00127.x
Rueda LM, Axtell RC (1985). In: Guide to common species of pupal parasites (Hymenoptera: Pteromalidae) of the house fly and other muscoid flies associated with poultry and livestock manure, Technical Bulletin 278. North Carolina Agricultural Research Service, North Carolina State University
Saragusty J, Hermes R, Hofer H, Bouts T, Goritz F, Hildebrandt TB (2012) Male pygmy hippopotamus influence offspring sex ratio. Nat Commun 3:697
Shuker DM, Sykes EM, Browning LE, Beukeboom LW, West SA (2006) Male influence on sex allocation in the parasitoid wasp Nasonia vitripennis. Behav Ecol Sociobiol 59:829–835. doi:10.1007/s00265-005-0129-1
Shuker DM, Phillimore AJ, Burton-Chellew MN, Hodge SE, West SA (2007) The quantitative genetic basis of polyandry in the parasitoid wasp, Nasonia vitripennis. Heredity 98:69–73
Shuker DM, Moynihan AM, Ross L (2009) Sexual conflict, sex allocation and the genetic system. Biol Lett 5:682–685. doi:10.1098/rsbl.2009.0427
Somjee U, Ablard K, Crespi B, Schaefer PW, Gries G (2011) Local mate competition in the solitary parasitoid wasp Ooencyrtus kuvanae. Behav Ecol Sociobiol 65:1071–1077. doi:10.1007/s00265-010-1114-x
Steiner S, Ruther J (2009) How important is sex for females of a haplodiploid species under local mate competition? Behav Ecol 20:570–574
Steiner S, Henrich N, Ruther J (2008) Mating with sperm-depleted males does not increase female mating frequency in the parasitoid Lariophagus distinguendus. Entomol Exp Appl 126:131–137
van Alphen J, Bernstein C, Driessen G (2003) Information acquisition and time allocation in insect parasitoids. Trends Ecol Evol 18:81–87
van den Assem J (1986) Mating behaviour in parasitic wasps. In: Waage J, Greathead D (eds) Insect parasitoids. Academic Press, New York, pp 137–163
van den Assem J, Jachmann F (1982) The coevolution of receptivity signalling and body-size dimorphism in the Chalcidoidea. Behavior 80:96–105
van den Assem J, Jachmann F, Simbolotti P (1980) Courtship behavior of Nasonia vitripennis (Hym, Pteromalidae): some qualitative, experimental evidence for the role of pheromones. Behavior 75:301–307
West SA (2009) Sex allocation. Princeton University Press, Princeton
Wild G, West SA (2009) Genomic imprinting and sex allocation. Am Nat 173:E1–E14. doi:10.1086/593305
Xu J, Wang Q (2010) Mechanisms of last male precedence in a moth: sperm displacement at ejaculation and storage sites. Behav Ecol 21:714–721
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Englewood Cliffs
Acknowledgments
We would like to thank A. Coletta, M. Hyett, J. Niew, T. Schulmeister, and A. van Pelt for assistance with experiments; J. Cooper and W. Nichols, Jr. for assistance with colony maintenance; B. Ball for the illustration; R. King and R. Moran for feedback on presentation; and K. Floate for U. rufipes. This research was supported by Northern Illinois University's Department of Biological Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Sakaluk
Rights and permissions
About this article
Cite this article
King, B.H., Kuban, K.A. Should he stay or should he go: male influence on offspring sex ratio via postcopulatory attendance. Behav Ecol Sociobiol 66, 1165–1173 (2012). https://doi.org/10.1007/s00265-012-1369-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1369-5