Abstract
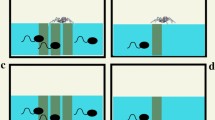
Animal aggregations are widespread in nature and can exhibit complex emergent properties not found at an individual level. We investigate one such example here, collective vortex formation by congeneric spadefoot toad tadpoles: Spea bombifrons and Spea multiplicata. Tadpoles of these species develop into either an omnivorous or a carnivorous (cannibalistic) morph depending on diet. Previous studies show that S. multiplicata are more likely to develop into omnivores and feed on suspended organic matter in the water body. The omnivorous morph is frequently social, forming aggregates that move and forage together and form vortices in which they adopt a distinctive slowly rotating circular formation. This behaviour has been speculated to act as a means to agitate the substratum in ponds and thus could be a collective foraging strategy. Here we perform a quantitative investigation of the behaviour of tadpoles within aggregates. We found that only S. multiplicata groups exhibited vortex formation, suggesting that social interactions differ between species. The probability of collectively forming a vortex, in response to introduced food particles, increased for higher tadpole densities and when tadpoles were hungry. Individuals inside a vortex moved faster and exhibited higher (by approximately 27%) tailbeat frequencies than those outside the vortex, thus incurring a personal energetic cost. The resulting environmental modification, however, suggests that vortex behaviour may be an adaptation to actively create and exploit a resource patch within the environment.





Similar content being viewed by others
References
Adrian RJ (1991) Particle-imaging techniques for experimental fluid mechanics. Annu Rev Fluid Mech 23:261–304
Adrian RJ (2005) Twenty years of particle image velocimetry. Exp Fluids 39:159–169
Arendt JD (2009) Influence of sprint speed and body size on predator avoidance in New Mexican spadefoot toads (Spea multiplicata). Behav Ecol 159:455–461
Arendt JD (2010) Morphological correlates of sprint swimming speed in five species of spadefoot toad tadpoles: comparison of morphometric methods. J Morphol 271:1044–1052
Arendt JD, Hoang L (2005) Effect of food level and rearing temperature on burst speed and muscle composition of western spadefoot toad (Spea hammondii). Funct Ecol 19:982–987
Bainbridge R (1958) The speed of swimming of fish as related to size and to the frequency and the amplitude of the tail beat. J Exp Biol 35:109–133
Baker CF, Montgomery JC (1999) The sensory basis of rheotaxis in the blind Mexican cave fish, Astynax fasciatus. J Comp Physiol A 184:519–527
Bazazi S, Romanczuk P, Thomas S, Schimansky-Geier L, Hale JJ, Miller GA, Sword GA, Simpson SJ, Couzin ID (2011) Nutritional state and collective motion: from individuals to mass migration. Proc R Soc B-Biol Sci 278(1704):356–363. doi:10.1098/rspb.2010.1447
Beauchamp G (1998) The effect of group size on mean food intake rate in birds. Biol Rev 73(4):449–472
Beauchamp G, Giraldeau L-A (1996) Group foraging revisited: information sharing or producer–scrounger game? Am Nat 148(4):738–743
Beauchamp G, Giraldeau L-A (1997) Patch exploitation in a producer–scrounger system: test of a hypothesis using flocks of spice finches (Lonchura punctulata). Behav Ecol 8(1):54–59
Beiswenger RE (1975) Structure and function in aggregations of tadpoles of the American toad, Bufo americanus. Herpetologica 31:222–233
Beiswenger RE (1977) Diel patterns of aggregative behavior in tadpoles of Bufo americanus, in relation to light and temperature. Ecology 58:98–108
Bertram B (1975) Social factors influencing reproduction in wild lions. J Zool 177:463–482
Biewener AA (2003) Animal locomotion. Oxford University Press, Oxford
Bonabeau E (1998) Social insect colonies as complex adaptive systems. Ecosystems 1:437–443
Bonabeau E, Theraulaz G, Deneubourg J-L, Franks NR, Rafelsberger O, Joly JL, Blanco S (1998) A model for the emergence of pillars, walls and royal chambers in termite nests. Philos Trans R Soc Lond B 353:1561–1576
Bragg AN (1965) Gnomes of the night: the spadefoot toads. University of Pennsylvania Press, Philadelphia
Buhl J, Sumpter DJT, Couzin ID, Hale JJ, Despland E, Miller ER, Simpson SJ (2006) From disorder to order in marching locusts. Science 312:1402–1406
Camazine S, Deneubourg J-L, Franks NR, Sneyd J, Theraulaz G, Bonabeau E (2001) Self-organization in biological systems. Princeton University Press, Princeton
Couzin ID (2007) Collective minds. Nature 445:715
Couzin ID (2009) Collective cognition in animal groups. Trends Cogn Sci 13(1):36–43
Couzin ID, Krause J (2003) Self-organization and collective behaviour of vertebrates. Adv Stud Behav 32:1–75
Couzin ID, Krause J, James R, Ruxton GD, Franks NR (2002) Collective memory and spatial sorting in animal groups. J Theor Biol 218:1–11
Couzin ID, Krause J, Franks NR, Levin SA (2005) Effective leadership and decision making in animal groups on the move. Nature 433:513–516
Creel S, Creel NM (1995) Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim Behav 50:1325–1339
Dall SRX (2005) Defining the concept of public information. Science 308:353–354
Danchin E, Giraldeau L-A, Valone TJ, Wagner RH (2004) Public information: from nosy neighbors to cultural evolution. Science 305:487–491
Daniel TL, Webb PW (1987) Physical determinants of locomotion. In: Dejours P, Bolis L, Taylor CR, Weibel ER (eds) Comparative physiology: life in water and on land. Liviana, New York, pp 343–369
Deneubourg J-L, Goss S, Franks NR, Pasteels JM (1989) The blind leading the blind: modelling chemically mediated army ant raid patterns. J Insect Behav 2:719–725
Duellman WE, Lescure J (1973) Life history and ecology of the hylid frog Osteocephalus taurinus, with observations on larval behavior. In: Occasional Papers of the Museum of Natural History, vol 13. University of Kansas, pp 1–12
Duellman WE, Trueb L (1994) Biology of amphibians. The John Hopkins University Press, Baltimore
Foster MS, McDiarmid RW (1982) Study of aggregative behaviour of Rhinophrynus dorsalis tadpoles: design and analysis. Herpetologica 38(3):395–404
Franks NR, Partridge LW (1993) Lanchester battles and the evolution of combat in ants. Anim Behav 45:197–199
Franks NR, Gomez N, Goss S, Deneubourg J-L (1991) The blind leading the blind in army ant raid patterns: testing a model of self-organisation (Hymenoptera: Formicidae). J Insect Behav 4:583–607
Giraldeau L-A, Caraco T (2000) Social foraging theory. Princeton University Press, Princeton
Gotwald WH (1995) Army ants: the biology of social predation. Cornell University Press, Ithaca, New York
Hansell M (2005) Animal architecture. Oxford University Press, Oxford
Helbing D, Keltsch J, Molnár P (1997a) Modelling the evolution of human trail systems. Nature 388:47–50
Helbing D, Schweitzer F, Keltsch J, Molnár P (1997b) Active walker model for the formation of human and animal trail systems. Phys Rev E 56:2527–2539
Hunter JR, Zweifel JR (1971) Swimming speed, tail beat frequency, tail beat amplitude, and size in jack mackerel, Trachurus symmetricus, and other fishes. Fish B-NOAA 69:253–266
Kanter MJ, Coombs S (2003) Rheotaxis and prey detection in uniform currents by Lake Michigan mottled sculpin (Cottus bairdi). J Exp Biol 206:59–70
Katz LC, Potel MJ, Wassersug RJ (1981) Structure and mechanisms of schooling in tadpoles of the clawed frog, Xenopus laevis. Anim Behav 29:20–23
King AJ, Isaac NJB, Cowlishaw G (2009) Ecological, social, and reproductive factors shape producer–scrounger dynamics in baboons. Behav Ecol 20(5):1039–1049
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
McDiarmid RW, Altig R (1999) Tadpoles: the biology of Anuran larvae. Chicago University Press, Chicago
Montgomery JC, Baker CF, Carton AG (1997) The lateral line can mediate rheotaxis in fish. Nature 389:960–963
Ordemann A, Balázsi G, Caspari E, Moss F (2003) Daphnia swarms: from single agent dynamics to collective vortex formation. P SPIE-IS&T ELECT IM 5110:172–179
Parrish JK, Edelstein-Keshet L (1999) Complexity, pattern, and evolutionary trade-offs in animal aggregations. Science 284:99–101
Petranka JW, Hayes L (1998) Chemically mediated avoidance of a predatory odonate (Anax junius) by American toad (Bufo americanus) and wood frog (Rana sylvatica) tadpoles. Behav Ecol Sociobiol 42:263–271
Pfennig DW (1990a) “Kin recognition” among spadefoot toad tadpoles: a side-effect of habitat selection? Evolution 44(4):785–798
Pfennig DW (1990b) The adaptive significance of an environmentally-cued developmental switch in an anuran tadpole. Oecologia (Berl) 85:101–107
Pfennig DW (1992) Proximate and functional causes of polyphenism in an anuran tadpole. Funct Ecol 6:167–174
Pfennig DW, Murphy PJ (2000) Character displacement in polyphenic tadpoles. Evolution 54:1738–1749
Pfennig DW, Mabry A, Orange D (1991) Environmental causes of correlations between age and size at metamorphosis in Scaphiopus multiplicata. Ecology 72(6):2240–2248
Pfennig DW, Reeve HK, Sherman PW (1993) Kin recognition and cannibalism in spadefoot toad tadpoles. Anim Behav 46:87–94
Pfennig KS, Chunco AJ, Lackey ACR (2007) Ecological selection and hybrid fitness: hybrids succeed on parental resources. Evol Ecol Res 9:341–354
Pomeroy LV (1981) Developmental polymorphism in the tadpoles of the spadefoot toad, Scaphiopus multiplicatus. University of California, Riverside
Powell GVN (1974) Experimental analysis of the social value of flocking by starlings (Sturnus vulgaris) in relation to predation and foraging. Anim Behav 22:501–505
Raffel M, Willert CE, Wereley ST, Kompenhans J (1998) Particle image velocimetry. Springer, Berlin
Richmond ND (1947) Life history of Scaphiopus holbrooki holbrooki (Harlan). Part I. Larval development and behavior. Ecology 28:53–67
Robertson DR, Sweatman HPA, Fletcher EA, Cleland MG (1976) Schooling as a mechanism for circumventing the territoriality of competitors. Ecology 57:1208–1220
Roche JP (1993) The benefits of kin recognition in tadpoles: a review of the literature. Maine Nat 1(2):13–20
Schlichting H, Gersten K (2000) Boundary-layer theory. Springer, Berlin
Schmidt RJ, Strand SW (1982) Cooperative foraging by yellowtail, Seriola lalandei (Carangidae) on two species of fish prey. Copeia 1982:714–717
Schmidt-Nielsen K (1975) Scaling in biology: the consequences of size. J Exp Zool 194(1):287–307
Schneirla TC (1971) Army ants: a study in social organisation. Freeman, San Francisco
Seeley TD (1995) The wisdom of the hive: the social physiology of honey bee colonies. Belknap Press of Harvard University Press, Cambridge
Simmons AM, Costa LM, Gerstein HB (2004) Lateral line-mediated rheotactic behavior in tadpoles of the African clawed frog (Xenopus laevis). J Comp Physiol A 190(9):747–758
Skelly D, Werner E (1990) Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71:2313–2322
Sokolov A, Apodacac MM, Grzybowskic BA, Aransona IS (2009) Swimming bacteria power microscopic gears. Proc Natl Acad Sci USA 107(3):969–974
Sontag C, Sloan Wilson D, Wilcox RS (2006) Social foraging in Bufo americanus tadpoles. Anim Behav 72:1451–1456
Stuart LC (1961) Some observations on the natural history of tadpoles of Rhinophrynus dorsalis Dumeril and Bibron. Herpetologica 17:73–79
Sumpter DJT (2006) The principles of collective animal behaviour. Philos Trans R Soc Lond B 361:5–22
Templeton JJ, Giraldeau L-A (1995) Patch assessment in foraging flocks of European starlings: evidence for the use of public information. Behav Ecol 6:65–72
Torney CJ, Neufeld Z, Couzin ID (2009) Context-dependent interaction leads to emergent search behavior in social aggregates. Proc Natl Acad Sci USA 106(52):22055–22060
Vickery WL, Giraldeau L-A, Templeton JJ, Kramer DL, Chapman CA (1991) Producers, scroungers and group foraging. Am Nat 137(6):847–863
Vogel S (2008) Modes and scaling in aquatic locomotion. Integr Comp Biol 48(6):702–712
Waldman B (1991) Kin recognition in amphibians. In: Hepper PG (ed) Kin recognition. Cambridge University Press, Cambridge, pp 162–219
Wassersug RJ (1973) Aspects of social behavior of anuran larvae. In: Vial JL (ed) Evolutionary biology of the anurans: contemporary research on major problems. University of Missouri Press, Columbia, pp 273–297
Wassersug RJ, Hoff K (1985) The kinematics of swimming in anuran larvae. J Exp Biol 119:1–30
Wells KD (2007) The ecology and behaviour of amphibians. Chicago University Press, Chicago
Acknowledgements
The authors acknowledge support from the Natural Environment Research Council (S.B.), Searle Scholars Award 08-SPP-201 (I.D.C.), National Science Foundation Awards PHY-0848755 (I.D.C.) and DEB-0542566 (K.S.P), Office of Naval Research Award N00014-09-1-1074 (I.D.C.) and a DARPA grant no. HR0011-09-1-0055 (to Princeton University). The authors also thank Laura Exline for help with the breeding of toads and laboratory assistance and Vishwesha Guttal, Arne Holmin, Christos Ioannou, Simon LeBlanc, Ryan Martin, Graham Taylor and Colin Torney for helpful discussions.
Ethical standards
The authors declared that all procedures were carried out in accordance with federal and state regulations and were approved by the University of North Carolina at Chapel Hill’s Institutional Animal Care and Use Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Krause
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 46.0 kb)
Rights and permissions
About this article
Cite this article
Bazazi, S., Pfennig, K.S., Handegard, N.O. et al. Vortex formation and foraging in polyphenic spadefoot toad tadpoles. Behav Ecol Sociobiol 66, 879–889 (2012). https://doi.org/10.1007/s00265-012-1336-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1336-1