Abstract
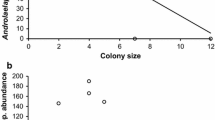
Social animals are susceptible to high infection levels by contact-transmitted parasites due to increased conspecific interaction. Exotic parasites are known to have adverse consequences on native hosts. We examined the relationship between social group size and exotic ectoparasite loads, and adult infection levels with per capita fitness and offspring survival in the plural breeding rodent Octodon degus in central Chile. Degus at our site were almost entirely infected by two exotic ectoparasites: the fleas Leptopsylla segnis and Xenopsylla cheopis. Neither group size nor number of females per group predicted the abundance of either exotic flea species. The per capita number of pups (per capita fitness) that emerged from burrow systems used by known social groups was negatively correlated with abundance of L. segnis but not X. cheopis. On adults, X. cheopis abundance was three times greater than L. segnis but was not significantly correlated with per capita fitness. In females, L. segnis abundance was negatively correlated with peak body mass during pregnancy. Adult ectoparasite load was not correlated with offspring survival. Based on these results, we hypothesize that high infection levels of L. segnis result in decreased reproductive fitness of adult female degus but are not a cost of sociality because parasite loads are not predicted by social group size. Further work is needed to experimentally test this hypothesis and to determine if L. segnis serves as a vector for a deleterious pathogen. Lastly, the lack of native ectoparasites may explain why a previous study at our site determined that behavioral adaptations needed to cope with high ectoparasite burdens (e.g., grooming) are not extensive in degus; they simply have not had the coevolutionary time needed for selection of these behaviors.


Similar content being viewed by others
References
Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Sys 5:325–383
Altizer S, Nunn CL, Thrall PH, Gittleman JL, Ezenwa V, Pulliam J, Pedersen AB, Dobson AP, Poss M, Cunningham A, Antonovics JL, Jones KE (2003) Social organization and disease risk in mammals: integrating theory and empirical studies. Annu Rev Ecol Evol Sys 34:517–547
Anderson RM, May RM (1982) Coevolution of hosts and parasites. Parasitology 85:411–426
Arnold W, Lichtenstein AV (1993) Ectoparasite loads decrease the fitness of alpine marmots (Marmota marmota) but are not a cost of sociality. Behav Ecol 4:36–39
Azad AF (1990) Epidemiology of murine typhus. Annu Rev Entomol 35:553–569
Becker MI, De Ioannes AE, León C, Ebensperger LA (2007) Females of communally breeding rodent, Octodon degus, transfer antibodies to their offspring during pregnancy and lactation. J Reprod Immunol 74:68–77
Blanco G, Tella JL, Potti J (1997) Feather mites on group-living red-billed choughs: a non-parasitic interaction? J Avian Biol 28:197–206
Brown CR, Brown MB (1986) Ectoparasitism as a cost of coloniality in cliff swallows (Hirundo pyrrhonata). Ecology 67:1206–1218
Burger JR, Chesh AS, Castro RA, Ortiz Tolhuysen L, Torre I, Ebensperger LA, Hayes LD (2009) The influence of trap type on evaluating population structure of the semifossorial and social rodent Octodon degus. Acta Theriol 54:311–320
Bush AO, Laffrey KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Castro DC, Cicchino A (2002) Las especies de los generos Gyropus Nitzsch, 1818 (Phthiraptera: Gyroponidae) parasites de Octodontidae (Mammalia:Rodentia). Rev Chil Hist Nat 75:293–298
Côté IM, Poulin R (1995) Parasitism and group size in social animals: a meta-analysis. Behav Ecol 6:159–165
Cully JF Jr, Williams ES (2001) Interspecific comparisons of sylvatic plague in prairie dogs. J Mamm 82:894–905
Davies CR, Ayres JM, Dye C, Deane LM (1991) Malaria infection rate of Amazonian primates increases with body weight and group size. Func Ecol 5:655–662
Dobson AP, Foufopoulos J (2001) Emerging infectious pathogens of wildlife. Phil Trans R Soc B 356:1001–1012
Ebensperger LA, Hurtado MJ (2005) Seasonal changes in the time budget of degus, Octodon degus. Behaviour 142:91–112
Ebensperger LA, Hurtado MJ, Soto-Gamboa M, Lacey EA, Chang AT (2004) Communal nesting and kinship in degus (Octodon degus). Naturwissenchaften 91:391–395
Ebensperger LA, Hurtado MJ, Valdivia I (2006) Lactating females do not discriminate between their own young and unrelated pups in the communally breeding rodent, Octodon degus. Ethology 112:921–929
Ebensperger LA, Hurtado MJ, León C (2007) An experimental examination of the consequences of communal versus solitary breeding on maternal condition and the early postnatal growth and survival of degu, Octodon degus, pups. Anim Behav 73:185–194
Ebensperger LA, Chesh AS, Castro RA, Ortiz Tolhuysen L, Quirici V, Burger JR, Hayes LD (2009) Instability rules social groups in the communal breeder rodent Octodon degus. Ethology 115:540–554
Freeland WJ (1976) Pathogens and the evolution of primate sociality. Biotropica 8:12–24
Freeland WJ (1979) Primate social groups as biological islands. Ecology 60:719–728
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Hart BL (1992) Behavioral adaptations to parasites: an ethological approach. J Parasitol 78:256–265
Hayes LD, Chesh AS, Ebensperger LA (2007) Ecological predictors of range areas and use of burrow systems in the diurnal rodent, Octodon degus. Ethology 113:155–165
Hayes LD, Chesh AS, Castro RA, Ortiz Tolhuysen L, Burger JR, Bhattacharjee J, Ebensperger LA (2009) Fitness consequences of group-living in the degu (Octodon degus), a plural breeder rodent with communal care. Anim Behav 78:131–139
Hayes LD, Burger JR, Soto-Gamboa M, Sobrero R, Ebensperger LA (2011) Towards an integrative model of caviomorph rodent sociality. J Mammal
Hillegass MA, Waterman JM, Roth JD (2008) The influence of sex and sociality on parasite loads in an African ground squirrel. Behav Ecol 19:1006–1011
Hillegass MA, Waterman JM, Roth JD (2010) Parasite removal increases reproductive success in a social African ground squirrel. Behav Ecol 21:696–700
Hoi H, Darolova A, König C, Kristofik J (1998) The relation between colony size, breeding density and ectoparasite loads of adult European bee-eaters (Merops apiaster). Ecoscience 5:156–163
Hoogland JL (1979) Aggression, ectoparasitism, and other possible costs of prairie dog (Sciuridae: Cynomys spp.) coloniality. Behaviour 69:1–35
Hoogland JL, Sherman P (1976) Advantages and disadvantages of bank swallow (Riparia riparia) coloniality. Ecol Mono 46:33–58
Johnson DDP, Stopka P, Macdonald DW (2004) Ideal flea constraints on group living: unwanted public goods and the emergence of cooperation. Behav Eco 15:181–186
Krasnov BR (2008) Functional and evolutionary ecology of fleas. Cambridge University Press, Cambridge
Lacey EA (2004) Sociality reduces individual direct fitness in a communally breeding rodent, the colonial tuco-tuco (Ctenomys sociabilis). Behav Ecol Sociobiol 56:449–457
Leroy EM, Rouquet P, Formentry P, Souquière S, Kilbourne A, Froment JM, Bermejo M, Smit S, Karesh W, Swanepoel R, Zaki SR, Rollin PE (2004) Multiple ebola virus transmission events and rapid decline of Central African wildlife. Science 303:387–390
Meserve PL, Martin RA, Rodriguez J (1984) Comparative ecology of the caviomorph rodent Octodon degus in two Chilean Mediterranean-type communities. Rev Chil Hist Nat 57:79–89
Møller AP, Dufva R, Allander K (1993) Parasites and the evolution of host social behavior. Advances in the Study of Behavior 22:65–102
Mooring MS, Hart BL (1992) Animal grouping for protection from parasites: selfish herd and encounter–dilution effects. Behaviour 123:173–193
Mooring MS, Hart BL, Fitzpatrick TA, Reisig DD, Nishihira TT, Fraser IC, Benjamin JE (2006) Grooming in desert bighorn sheep (Ovis canadensis mexicana) and the ghost of parasites past. Behav Ecol 17:364–371
Newman MEJ (2004) Analysis of weighted networks. Phys Rev E 70:056131
O'Brien TG (1993) Allogrooming behaviour among adult female wedge-capped capuchin monkeys. Anim Behav 46:499–510
Pedersen AB, Fenton A (2007) Emphasizing the ecology in parasite community ecology. Trends Ecol Evo 22:133–139
Perez-Orella C, Shulte-Holstedde AI (2005) Effects of sex and body size on ectoparasite loads in the northern flying squirrel (Glaucomys sabrinus). Can J Zool 83:1381–1385
Poulin R (1991) Group-living and infestation by ectoparasites in passerines. Condor 93:418–423
Price RD, Timm RM (2000) Review of the chewing louse genus Abrocomophaga (Phthiraptera: Amblycera), with description of two new species. Proc Biol Soc Wash 113:210–217
Quan YF, Macmanes MD, Ebensperger LA, Lacey EA, Hayes LD (2009) Isolation and characterization of polymorphic microsatellite loci from Octodon degus. Molecular Ecology Resources 9:1000–1001
Roelke-Parker ME, Munson L, Packer C, Kock R, Cleaveland S, Carpenter M, O'Brien SJ, Pospischil A, Hofmann-Lehmann R, Lutz H, Mwamengele GLM, Mgasa MN, Machange GA, Summers BA, Appel MJG (1996) A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 379:441–445
Roulin A, Heeb P (1996) The immunological function of allosuckling. Ecol Lett 2:319–324
Rubenstein DI, Hohmann ME (1989) Parasites and social behavior of island feral horses. Oikos 55:300–312
Silk JB (2007) The adaptive value of sociality in mammalian groups. Phil Tran R Soc B 362:539–559
Stapp P, Antolin MF, Ball M (2004) Patterns of extinction in prairie dog metapopulations: plague outbreaks follow El Niño events. Front Ecol Environ 2:235–240
Stopka P, Graciasova R (2001) Conditional allo-grooming in the herb field mouse. Behav Ecol 12:584–589
The Animal Care and Use Committee of the American Society of Mammalogists (2007) Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 88:809–823
Thorne ET, Williams E (1988) Disease and endangered species: the black-footed ferret as a recent example. Con Bio 2:66–74
Van Vuren D (1996) Ectoparasites, fitness, and social behavior of yellow-bellied marmots. Ethology 102:686–694
Veloso C, Kenagy GJ (2005) Temporal dynamics of milk composition of the precocial caviomorph Octodon degus (Rodentia: Octodontidae). Rev Chil Hist Nat 78:247–252
Waterman JM (2002) Delayed maturity, group fission and the limits of group size in Cape ground squirrels. (Sciuridae: Xerus inauris). J Zool 256:113–120
Whitehead H (2009) SOCPROG programs: analyzing animal social structures. Behav Ecol Sociobiol 63:765–778
Whiteman NK, Parker PG (2004) Effects of host sociality on ectoparasite population biology. J Parasitol 90:939–947
Acknowledgements
We thank Universidad de Chile, in particular, former field station administrator J.D. Garcia, for help in facilitating fieldwork, V. Quirici, C. León, R. Castro, L. Ortiz Tolhuysen, M. Pardue, N. Schaferkotter, and J. Childers for field assistance, and A. Edelman and R. Fiorillo for helpful comments on this manuscript. This work was funded by the National Science Foundation EPSCoR (#0553910), the Louisiana Board of Regents Research Competitiveness Program (#LEQSF 2007–2009-RD-A-39), The University of Louisiana at Monroe HHMI Program and the ULM Office of Academic Affairs, the Sigma Xi Scientific Society, the American Society of Mammalogists, FONDECYT grants 1020861 and 1060499 and by Centro de Estudios Avanzados en Ecología y Biodiversidad (FONDAP 1501-001). The authors declare no conflict of interest. Field procedures followed guidelines established by the American Society of Mammalogists Animal Care and Use Committee, were approved by the ULM Institutional Animal Care and Use Committee, and are in accordance with U.S. and Chilean laws (permit no. 1-58.205 [2711] by Servicio Agrícola y Ganadero).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Brown
Rights and permissions
About this article
Cite this article
Burger, J.R., Chesh, A.S., Muñoz, P. et al. Sociality, exotic ectoparasites, and fitness in the plural breeding rodent Octodon degus . Behav Ecol Sociobiol 66, 57–66 (2012). https://doi.org/10.1007/s00265-011-1252-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1252-9