Abstract
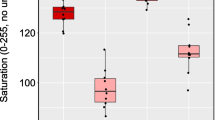
Plumage coloration, particularly when carotenoid-based, is important in social signaling in birds. Although feather color is a relatively stable trait, individuals may modify it with “cosmetic” substances such as preen oils. In addition, dirt accumulation may influence plumage coloration and further affect signal perception by receivers. Here, we analyze the separate potential effects of preen oils and soil accumulation on the reflectance properties of carotenoid-pigmented feathers across the visual range of most bird species, which includes the ultraviolet (UV). Using the yellow portion of tail feathers of Bohemian waxwings (Bombycilla garrulus), we performed two separate experiments where: (a) preen oils and/or soil were removed, or (b) preen oils (from black-billed magpies Pica pica or eagle owls Bubo bubo) were added. Preen oil addition reduced brightness but increased UV hue and yellow chroma. UV chroma was reduced by the addition of magpie (but not owl) preen oil. Soil accumulation had little effect on plumage reflectance in the UV range but significantly reduced yellow chroma. According to models of avian vision, both of these effects are detectable by birds and biologically meaningful when compared with natural variation between the sexes and age classes. We conclude that preen oil and soil accumulation can significantly affect the UV–visible reflectance of carotenoid-based plumages. As such traits typically advertise individual quality, preening and soiling have the potential to modify the information content of carotenoid-based plumage traits and how these signals are perceived by receivers.




Similar content being viewed by others
References
Andersson S, Prager M (2006) Quantifying colors. In: Hill EG, McGraw KJ (eds) Bird coloration, vol 1: mechanism and measurements. Harvard University Press, Cambridge, pp 41–89
Avilés JM, Soler JJ (2009) Nestling coloration is adjusted to parent visual performance in altricial birds. J Evol Biol 22:376–386
Bleiweiss R (2005) Variation in ultraviolet reflectance by carotenoid bearing feathers of tanagers (Thraupini: Emberizinae: Passeriformes). Biol J Linn Soc 84:243–257
Bowmaker JK, Martin GR (1978) Visual pigments and colour vision in a nocturnal bird, Strix aluco (tawny owl). Vis Res 18:1125–1130
Burtt EH, Ichida JM (1999) Occurrence of feather-degrading bacilli in the plumage of birds. Auk 116:364–372
Cuthill IC (2006) Color perception. In: Hill EG, McGraw KJ (eds) Bird coloration, vol 1: mechanism and measurements. Harvard University Press, Cambridge, pp 3–40
Delhey K, Peters A, Kempenaers B (2007) Cosmetic coloration in birds: occurrence, function, and evolution. Am Nat 169:S145–S158
Delhey K, Peters A, Biedermann PHW, Kempenaers B (2008) Optical properties of the uropygial gland secretion: no evidence for UV cosmetics in birds. Naturwissenschaften 95:939–946
Faivre B, Grégoire A, Préault M, Cézilly F, Sorci G (2003) Immune activation rapidly mirrored in a secondary sexual trait. Science 300:103
Figuerola J, Senar JC (2005) Seasonal changes in carotenoid- and melanin-based plumage coloration in great tit Parus major. Ibis 147:797–802
Folstad I, Karter AJ (1992) Parasites, bright males and the immunocompetence handicap. Am Nat 139:603–622
Galván I, Amo L, Sanz JJ (2008) Ultraviolet-blue reflectance of some nestling plumage patches mediates parental favouritism in great tits Parus major. J Avian Biol 39:277–282
Ghosh A, Bhattacharyya SP (1996) Endocrine factors in the regulation of the activity of the uropygial gland of avian skin. Proc Indian Natl Sci Acad B Biol Sci 66:227–236
Goldstein DL (1988) Estimates of daily energy expenditure in birds: the time-energy budget as an integrator of laboratory and field studies. Am Zool 28:829–844
Gomez D (2006) AVICOL, a program to analyse spectrometric data. Free program available at http://sites.google.com/site/avicolprogram/ or from the author at dodogomez@yahoo.fr. Accessed 14 June 2010
Hart NS (2002) Vision in the peafowl (Aves: Pavo cristatus). J Exp Biol 205:3925–3935
Hart NS, Hunt DM (2007) Avian visual pigments: characteristics, spectral tuning, and evolution. Am Nat 169:S7–S26
Hart NS, Partridge JC, Cuthill IC, Bennett ATD (2000) Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J Comp Physiol A 186:375–387
Hausmann F, Arnold KE, Marshall NJ, Owens IPF (2003) Ultraviolet signals in birds are special. Proc R Soc Lond B 270:61–67
Hill EG, McGraw KJ (eds) (2006a) Bird coloration, vol 2: function and evolution. Harvard University Press, Cambridge
Hill EG, McGraw KJ (eds) (2006b) Bird coloration, vol 2: mechanisms and measurements. Harvard University Press, Cambridge
Hudon J, Brush AH (1989) Probable dietary basis of a color variant of the cedar waxwing. J Field Ornithol 60:361–368
Isaksson C, Örnborg J, Prager M, Andersson S (2008) Sex and age differences in reflectance and biochemistry of carotenoid-based colour variation in the great tit Parus major. Biol J Linn Soc 95:758–765
Jacob J, Ziswiler V (1982) The uropygial gland. In: Farner DS, King JR, Parkes KC (eds) Avian biology, vol VI. Academic, New York, pp 199–324
Lenouvel P, Gomez D, Théry M, Kreutzer M (2009) Do grooming behaviours affect properties of feathers in male domestic canaries, Serinus canaria? Anim Behav 77:1253–1260
López-Rull I, Pagán I, Macías García C (2010) Cosmetic enhancement of signal coloration: experimental evidence in the house finch. Behav Ecol 21:781–787
Martínez-Padilla J, Mougeot F, Pérez-Rodríguez L, Bortolotti GR (2007) Nematode parasites reduce carotenoid-based signalling in male red grouse. Biol Lett 3:161–164
Mays HL, McGraw KJ, Ritchison G, Cooper S, Rush V, Parker RS (2004) Sexual dichromatism in the yellow-breasted chat Icteria virens: spectrophotometric analysis and biochemical basis. J Avian Biol 35:125–134
McGraw KJ (2006) Mechanics of carotenoid-based coloration. In: Hill EG, McGraw KJ (eds) Bird coloration. vol 1: mechanism and measurements. Harvard University Press, Cambridge, pp 177–242
McGraw KJ, Hill GE (2004) Plumage color as a dynamic trait: carotenoid pigmentation of male house finches Carpodacus mexicanus fades during the breeding season. Can J Zool 82:734–738
Montgomerie R (2006a) Cosmetic and adventitious colors. In: Hill EG, McGraw KJ (eds) Bird coloration, vol 1: mechanism and measurements. Harvard University Press, Cambridge, pp 399–427
Montgomerie R (2006b) Analyzing colors. In: Hill EG, McGraw KJ (eds) Bird coloration, vol 1: mechanism and measurements. Harvard University Press, Cambridge, pp 90–147
Montgomerie R, Lyon B, Holder K (2001) Dirty ptarmigan: behavioral modification of conspicuous male plumage. Behav Ecol 12:429–438
Mountjoy DJ (2005) Family Bombycillidae (Waxwings). In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the birds of the world, vol 10: Cuckoo-shrikes to thrushes. Lynx Edicions, Barcelona, pp 304–318
Negro JJ, Margalida A, Hiraldo F, Heredia R (1999) The function of the cosmetic coloration of bearded vultures: when art imitates life. Anim Behav 58:F14–F17
Ödeen A, Håstad O (2003) Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol Biol Evol 20:855–861
Oka N, Okuyama M (2000) Nutritional status of dead oiled rhinoceros auklets (Cerorhinca monocerata) in the southern Japan sea. Mar Pollut Bull 40:340–347
Örnborg J, Andersson S, Griffith SC, Sheldon BC (2002) Seasonal changes in a ultraviolet structural colour signal in blue tits, Parus caeruleus. Biol J Linn Soc Lond 76:237–245
Parejo D, Avilés JM, Rodríguez J (2010) Visual cues and parental favouritism in a nocturnal bird. Biol Lett 6:171–173
Pérez-Rodríguez L (2008) Carotenoid-based ornamentation as a dynamic but consistent individual trait. Behav Ecol Sociobiol 62:995–1005
Piersma T, Dekker M, Damste JSS (1999) An avian equivalent of make-up? Ecol Lett 2:201–203
Reneerkens J, Korsten P (2004) Plumage reflectance is not affected by preen wax composition in red knots Calidris canutus. J Avian Biol 35:405–409
Saks L, McGraw KJ, Hõrak P (2003) How feather colour reflects its carotenoid content. Funct Ecol 17:555–561
Shawkey MD, Hill GE (2005) Carotenoids need structural colours to shine. Biol Lett 1:121–124
Shawkey MD, Pillai SR, Hill GE (2003) Chemical warfare? Effects of uropygial oil on feather-degrading bacteria. J Avian Biol 34:345–349
Shawkey MD, Hill GE, McGraw KJ, Hood WR, Huggins K (2006) An experimental test of the contributions and condition-dependence of microstructure and carotenoids in yellow plumage colouration. Proc R Soc Lond B 273:2985–2991
Sheldon BC, Andersson S, Griffith SC, Örnborg J, Sendecka J (1999) Ultraviolet colour variation influences blue tit sex ratios. Nature 402:874–877
Siefferman L, Hill GE (2005) UV-blue structural coloration and competition for nest boxes in male eastern bluebirds. Anim Behav 69:67–72
Surmacki A (2008) Preen waxes do not protect carotenoid plumage from bleaching by sunlight. Ibis 150:335–341
Surmacki A, Nowakowski JK (2007) Soil and preen waxes influence the expression of carotenoid-based plumage colouration. Naturwissenschaften 94:829–835
Vorobyev M (2003) Coloured oil droplets enhance colour discrimination. Proc R Soc Lond B 270:1255–1261
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B 265:351–358
Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC (1998) Tetrachromacy, oil droplets and bird plumage colours. J Comp Physiol A 183:621–633
Walther BA, Clayton DH (2005) Elaborate ornaments are costly to maintain: evidence for high maintenance handicaps. Behav Ecol 16:89–95
Zahavi A, Zahavi A (1997) The handicap principle. A missing piece of Darwin’s puzzle. Oxford University Press, Oxford
Zampiga E, Hoi H, Pilasto A (2004) Preening, plumage reflectance and female choice in budgerigars. Ethol Ecol Evol 16:339–349
Acknowledgments
We are grateful to Elenis Crespo and Raspa de Prada (Centro de Recuperación de Fauna Silvestre “El Chaparrillo”) for their help during preen oil collection from eagle owls. Doris Gomez and Jesús M. Avilés kindly provided advice on some computational details of visual models. We also thank Jesús T. García for genetic sexing of the birds and Ismael Galván and two anonymous referees for their comments on the manuscript. L. P.-R. was supported by a postdoctoral contract (07/028-A) from Junta de Comunidades de Castilla-La Mancha (JCCM) and a “Juan de la Cierva” contract from the Spanish Ministerio de Ciencia e Innovación (MICINN). Funding was also provided by Natural Sciences and Engineering Research Council of Canada and the Stuart and Mary Houston Professorship in Ornithology (to G. R. B).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Fernandez-Juricic
Rights and permissions
About this article
Cite this article
Pérez-Rodríguez, L., Mougeot, F. & Bortolotti, G.R. The effects of preen oils and soiling on the UV–visible reflectance of carotenoid-pigmented feathers. Behav Ecol Sociobiol 65, 1425–1435 (2011). https://doi.org/10.1007/s00265-011-1153-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1153-y