Abstract
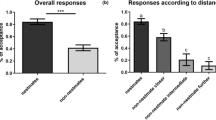
Hosts of the common cuckoo (Cuculus canorus), an avian brood parasite, develop antiparasite defense mechanisms to increase their reproductive success. Ejection of the parasite egg and desertion of the parasitized nest are the most typical adaptations in response to brood parasitism, but nest desertion may also occur in response to partial clutch reduction, independently from parasitism. Some great reed warblers (Acrocephalus arundinaceus) showed both mechanisms in the same incidence of cuckoo parasitism: in 18% of successful ejections of the parasite eggs, they deserted their nests. We studied if such cases of post-ejection nest-desertion are caused by brood parasitism or reduced clutch value. We experimentally parasitized clutches consisting of five or three host eggs with two painted conspecific eggs to mimic parasitic eggs, as multiple parasitism is frequent in the area. Although hosts ejected these parasitic eggs in both clutch categories (100% and 67% for the larger and smaller inital clutch sizes, respectively), we found that after manipulation, post-ejection nest-desertion frequently occurred at small (3-egg) clutches (40%), but rarely at large (5-egg) clutches (17%). The same phenomenon also occurred when unparasitized 3-egg clutches were reduced by two eggs, but not when 5-egg clutches were reduced in the same way. A logistic regression model revealed that only initial clutch size affected nest desertion of parasitized nests in our experiments. Therefore, we conclude that post-ejection nest-desertion is not a second antiparasite mechanism, which might serve as a redundant antiparasite defense, but a reaction to typically small and further decreased clutch size.

Similar content being viewed by others
References
Alvarez F (1994) A gens of cuckoo Cuculus canorus parasitizing rufous bush chat Cercotrichas galactotes. J Avian Biol 25:239–243
Alvarez F (1999) Attractive non-mimetic stimuli in cuckoo Cuculus canorus eggs. Ibis 141:142–144
Anderson DR (2008) Model based inference in the life sciences: a primer on evidence. Springer, New York
Antonov A, Stokke BG, Moksnes A, Kleven O, Honza M, Røskaft E (2006) Eggshell strength of an obligate brood parasite: a test of the puncture resistance hypothesis. Behav Ecol Sociobiol 60:11–18
Antonov A, Stokke BG, Moksnes A, Røskaft E (2007) First evidence of regular common cuckoo, Cuculus canorus, parasitism on eastern olivaceous warblers, Hippolais icterina elaeica. Naturwiss 94:307–312
Antonov A, Stokke BG, Moksnes A, Røskaft E (2008) Does the cuckoo benefit from laying unusually strong eggs? Anim Behav 76:1893–1900
Bártol I, Karcza Z, Moskát C, Røskaft E, Kisbenedek T (2002) Responses of great reed warblers Acrocephalus arundinaceus to experimental brood parasitism: the effects of a cuckoo Cuculus canorus dummy and egg mimicry. J Avian Biol 33:420–425
Brooke M de L, Davies NB (1988) Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 35:630–632
Burnham KP, Anderson DR (2002) Model selection and multimodal inference. A practical information-theoretic approach. Springer, New York
Davies NB (2000) Cuckoos, cowbirds and other cheats. T. & A.D. Poyser, London
Davies NB, Brooke M de L (1988) Cuckoos versus reed warblers: adaptations and counteradaptations. Anim Behav 36:262–284
Dawkins NB, Krebs JR (1979) Arms races between and within species. Proc R Soc Lond B 205:489–511
Dyrcz A, Halupka K (2007) Why does the frequency of nest parasitism by the cuckoo differ considerably between two populations of warblers living in the same habitat? Ethology 113:200–213
Guigueno MF, Sealy SG (2009) Nest sanitation plays a role in egg burial by yellow warblers. Ethology 115:247–256
Hansson B, Bensch S, Hasselquist D (1997) Infanticide in great reed warblers: secondary females destroy eggs of primary females. Anim Behav 54:297–304
Hargitai R, Moskát C, Bán M, Gil D, López-Rull I, Solymos E (2010) Eggshell characteristics and yolk composition in the common cuckoo Cuculus canorus: are they adapted to brood parasitism? J Avian Biol 41:177–185
Hauber ME, Sherman PW (2001) Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci 24:609–616
Hauber ME, Moskát C, Bán M (2006) Experimental shift in hosts' acceptance threshold of inaccurate-mimic brood parasite eggs. Biol Lett 2:177–180
Higuchi H (1998) Host use and egg color of Japanese cuckoos. In: Rothstein SI, Robinson SK (eds) Parasitic birds and their hosts. Studies in coevolution. Oxford University Press, New York, pp 80–93
Hill DE, Sealy SG (1994) Desertions of nests parasitized by cowbirds: have clay-coloured sparrows evolved an anti-parasite defence? Anim Behav 48:1063–1070
Honza M, Moskát C (2005) Antiparasite behaviour in response to experimental brood parasitism in the great reed warbler: a comparison of single and multiple parasitism. Ann Zool Fenn 42:627–633
Honza M, Moskát C (2008) Egg rejection behaviour in the great reed warbler (Acrocephalus arundinaceus): the effect of egg type. J Ethol 26:389–395
Honza M, Picman J, Grim T, Novák V, Capek MJr, Mrlik V (2001) How to hatch from an egg of great structural strength. A study of the common cuckoo. J Avian Biol 32:249–255
Honza M, Procházka P, Stokke BG, Moksnes A, Røskaft E, Capek MJr, Mrlík V (2004) Are blackcaps current winners in the evolutionary struggle against the common cuckoo? J Ethol 22:175–180
Honza M, Kuiper SM, Cherry MI (2005) Behaviour of African turdid hosts towards experimental parasitism with artificial red-chested cuckoo Cuculus solitarius eggs. J Avian Biol 36:517–522
Hosoi SA, Rothstein SI (2000) Nest desertion and cowbird parasitism: evidence for evolved responses and evolutionary lag. Anim Behav 59:823–840
Kemal RE, Rothstein SI (1988) Mechanisms of avian egg recognition: adaptive responses to eggs with broken shells. Anim Behav 36:175–183
Kilner RM (2006) The evolution of egg colour and patterning in birds. Biol Rev 81:383–406
Kosciuch KL, Parker TH, Sandercock BK (2006) Nest desertion by a cowbird host: an antiparasite behavior or a response to egg loss? Behav Ecol 17:917–924
Krüger O (2007) Cuckoos, cowbirds and hosts: adaptations, trade-offs and constraints. Phil Trans R Soc B 362:1873–1886
Lahti DC, Lahti AR (2002) How precise is egg discrimination in weaverbirds? Anim Behav 63:1135–1142
Lee J-W, Yoo J-C (2004) Effect of host egg color dimorphism on interactions between the vinous-throated parrotbill (Paradoxornis webbianus) and common cuckoo (Cuculus canorus). Korean J Biol Sci 8:77–80
Lotem A, Nakamura H, Zahavi A (1992) Rejection of cuckoo eggs in relation to host age: a possible evolutionary equilibrium. Behav Ecol 3:128–132
Lotem A, Nakamura H, Zahavi A (1995) Constraints on egg discrimination and cuckoo-host co-evolution. Anim Behav 49:1185–1209
Lovászi P, Moskát C (2004) Break-down of arms race between the red-backed shrike (Lanius collurio) and common cuckoo (Cuculus canorus). Behaviour 141:245–262
Moksnes A, Røskaft E (1995) Egg-morph and host preferences in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J Zool Lond 236:625–648
Moksnes A, Røskaft E, Braa AT, Korsnes L, Lampe H, Pedersen HC (1991) Behavioural responses of potential hosts towards artificial cuckoo eggs and dummies. Behaviour 116:64–89
Moksnes A, Røskaft E, Korsnes L (1993) Rejection of cuckoo (Cuculus canorus) eggs by meadow pipits (Anthus pratensis). Behav Ecol 4:120–127
Moskát C, Honza M (2002) European cuckoo Cuculus canorus parasitism and host's rejection behaviour in a heavily parasitized great reed warbler Acrocephalus arundinaceus population. Ibis 144:614–622
Moskát C, Hauber ME (2007) Conflict between egg recognition and rejection decisions in common cuckoo (Cuculus canorus) hosts. Anim Cogn 10:377–386
Moskát C, Székely T, Kisbenedek T, Karcza Z, Bártol I (2003) The importance of nest cleaning in egg rejection behaviour of great reed warblers Acrocephalus arundinaceus. J Avian Biol 34:16–19
Moskát C, Hansson B, Barabás L, Bártol I, Karcza Z (2008a) Common cuckoo Cuculus canorus parasitism, antiparasite defence and gene flow in closely located populations of great reed warblers Acrocephalus arundinaceus. J Avian Biol 39:663–671
Moskát C, Székely T, Cuthill IC, Kisbenedek T (2008b) Hosts' responses to parasitic eggs: which cues elicit hosts' egg discrimination? Ethology 114:186–194
Moskát C, Hauber ME, Avilés JM, Bán M, Hargitai R, Honza M (2009) Increased host tolerance of multiple cuckoo eggs leads to higher fledging success of the brood parasite. Anim Behav 77:1281–1290
Moskát C, Bán M, Székely T, Komdeur J, Lucassen RWG, van Boheemen LA, Hauber ME (2010) Discordancy or template-based recognition? Dissecting the cognitive basis of the rejection of foreign eggs in hosts of avian brood parasites. J Exp Biol 213:1976–1983
Ortega CP, Cruz A (1988) Mechanisms of egg acceptance by marsh-dwelling blackbirds. Condor 90:349–358
Payne R (2005) Cuckoos, Cuculidae. Oxford University Press, Oxford
Pianka ER (1976) Natural selection of optimal reproductive tactics. Am Zool 16:775–784
Pozgayová M, Procházka P, Honza M (2009) Sex-specific defence behaviour against brood parasitism in a host with female-only incubation. Behav Process 81:34–38
Rothstein SI (1974) Mechanisms of avian egg recognition: possible learned and innate factors. Auk 91:796–807
Rothstein SI (1975) An experimental and teleonomic investigation of avian brood parasitism. Condor 77:250–271
Rothstein SI (1982) Success and failures in avian egg and nestling recognition with comments on the utility of optimality reasoning. Amer Zool 22:547–560
Rothstein SI (1986) A test of optimality: egg recognition in the eastern phoebe. Anim Behav 34:1109–1119
Rothstein SI, Robinson SK (1998) The evolution and ecology of brood parasitism. In: Rotstein SI, Robinson SK (eds) Parasitic birds and their hosts: studies in coevolution. Oxford University Press, New York, pp 3–56
Sargent RC, Gross MR (1985) Parental investment decision rules and the Concorde fallacy. Behav Ecol Sociobiol 17:43–45
Servedio M, Hauber ME (2006) To eject or to abandon? Life history traits of hosts and parasites interact to influence the fitness payoffs of alternative anti-parasite strategies. J Evol Biol 19:1585–1594
Stokke BG, Honza M, Moksnes A, Røskaft E, Rudolfsen G (2002) Costs associated with recognition and rejection of parasitic eggs in two European passerines. Behaviour 139:629–644
Stokke BG, Rudolfsen G, Moksnes A, Røskaft E (2004) Rejection of conspecific eggs in chaffinches: the effect of age and clutch characteristics. Ethology 110:459–470
Strausberger BM, Burhans DE (2001) Nest desertion by field sparrows and its possible influence on the evolution of cowbird behavior. Auk 118:770–776
Svennungsen TO, Holen OH (2010) Avian brood parasitism: information use and variation in egg-rejection behaviour. Evolution 64:1459–1469
Székely T, Webb JN, Houston AI, McNamara JM (1996) An evolutionary approach to offspring desertion in birds. Curr Ornithol 13:271–330
Takasu F (1998) Modelling the arms race in avian brood parasitism. Evol Ecol 12:969–987
Takasu F, Moskát C, Munoz AR, Imanishi S, Nakamura H (2009) Adaptations in the common cuckoo (Cuculus canorus) to host eggs in a multiple-hosts system of brood parasitism. Biol J Linn Soc 98:291–300
Trnka A, Prokop P, Batáry P (2010) Infanticide or interference: does the great reed warbler selectively destroy eggs? Ann Zool Fennici 47:272–277
Underwood TJ, Sealy SG (2006) Influence of shape on egg discrimination in American robins and gray catbirds. Ethology 112:164–173
Valera F, Hoi H, Schleicher B (1997) Egg burial in Penduline Tits Remiz pendulinus: its role in nest desertion and female polyandry. Behav Ecol 8:20–27
van Dijk RE, Szentirmai I, Komdeur J, Székely T (2007) Sexual conflict over parental care in Penduline Tits Remiz pendulinus: the process of clutch desertion. Ibis 149:530–534
Winkler DW (1991) Parental investment decision rules in tree swallows: parental defense, abandonment, and the so-called Concorde Fallacy. Behav Ecol 2:133–142
Wyllie I (1981) The cuckoo. Batsford, London
Acknowledgments
The study was supported by the Office of Academy-supported Research Groups Affiliated with Universities and Other Institutions of the Hungarian Academy of Sciences (to C.M.) and by the Groningen University Grant (to E.R. and M.B.). Timea Protovin, Michael G. Anderson, and István Zsoldos kindly helped in the fieldwork. We thank Hannah Dugdale (Groningen University, Netherlands/Sheffield University, UK) for her valuable comments on the manuscript. The Middle-Danube-Valley Inspectorate for Environmental Protection, Nature Conservation and Water Management provided permission for research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Soler
Rights and permissions
About this article
Cite this article
Moskát, C., Rosendaal, E.C., Boers, M. et al. Post-ejection nest-desertion of common cuckoo hosts: a second defense mechanism or avoiding reduced reproductive success?. Behav Ecol Sociobiol 65, 1045–1053 (2011). https://doi.org/10.1007/s00265-010-1109-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-1109-7