Abstract
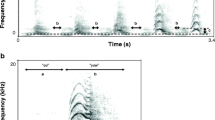
Facultative traits that have evolved under sexual selection, such as the acoustic ornaments present in the advertisement signals of male túngara frogs (Physalaemus pustulosus), offer a unique opportunity to examine selection for trait exaggeration with a focus on individual differences amongst signalers. By contrast, many studies of mate choice use experimental designs that obscure the inter-individual variation amongst signalers available for selection to act on—through the use of “typical” or average signals from the population. Here, we use dichotomous female phonotaxis choice tests to determine how the value of male call embellishment varies across 20 individual males frogs recorded from the wild—a sample which captures the acoustic diversity present in the population. We tested 20 females for each male call pair (i.e., 400 females). The results show widespread preference amongst females for ornamented calls (“whine–chucks”) over simple calls (“whines”), yet also demonstrate substantial variation in the relative benefits for individual male frogs—some males enjoy appreciable benefits by using ornaments while others (30% of males in this study) do not. We also show that the relative amplitude of the chuck to the whine correlates positively with the value of call elaborations across these 20 males. Finally, by manipulating the relative amplitude of whines and chucks using both natural and synthetic calls, we demonstrate directly that this single call parameter is key to determining the relative value of call elaborations across males.





Similar content being viewed by others
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Baugh AT, Ryan MJ (2010) Mate choice in response to dynamic presentation of male advertisement signals in túngara frogs. Anim Behav 79:145–152
Bernal X, Rand AS, Ryan MJ (2006) Acoustic preferences and localization performance of blood-sucking flies (Corethella Coquillett). Behav Ecol 17:709–715
Bernal XE, Akre KL, Baugh AT, Rand AS, Ryan MJ (2009) Female and male behavioral response to advertisement calls of graded complexity in túngara frogs, Physalaemus pustulosus. Behav Ecol Sociobiol 63:1269–1279
Cummings ME, Mollaghan DM (2006) Repeatability and consistency of female preference behaviours in a northern swordtail, Xiphophorus nigrensis. Anim Behav 72:217–224
Cummings ME, Rosenthal GG, Ryan MJ (2003) A private ultraviolet channel in visual communication. Proc Roy Soc Lond B 270:897–904
Dale J (2006) Intraspecific variation in coloration. In: Hill GE, McGraw KJ (eds) Bird coloration: volume 2, function and evolution. Harvard University Press, Cambridge, pp 36–86
Darwin C (1871) The descent of man, and selection in relation to sex. Murray, London
Dochtermann NA (2010) Behavioral syndromes: carryover effects, false discovery rates, and a prior hypotheses. Behav Ecol 21:437–439
Espmark Y (1995) Individual and local variations in the song of the snow bunting Plectrophenax nivalis on Spitzbergen. Bioacoustics 6:117–134
Gerhardt HC (1978) Discrimination of intermediate sounds in a synthetic call continuum by female green tree frogs. Science 199:1089–1091
Gerhardt HC, Schwartz JJ (2001) Auditory tuning and frequency preferences in anurans. In: Ryan MJ (ed) Anuran communication. Smithsonian, Washington, D.C., pp 73–85
Gerhardt HC, Brooks R (2009) Experimental analysis of multivariate female choice in gray treefrogs (Hyla versicolor): evidence for directional and stabilizing selection. Evolution 63:2504–2512
Hill GE (1991) Plumage coloration is a sexually selected indicator of male quality. Nature 350:337–339
Jang Y, Greenfield MD (1998) Absolute versus relative measures of sexual selection: assessing the contributions of ultrasonic signal characters to mate attraction in lesser wax moths, Achroia grisella (Leptidopters: Pyralidae). Evolution 52:1383–1393
Kodric-Brown A (1988) Effects of sex-ratio manipulation on territoriality and spawning success of the male pupfish, Cyprinodon pecosensis. Anim Behav 36:1136–1144
McClintock WJ, Uetz GW (1996) Female choice and pre-existing bias: visual cues during courtship in two Schizocosa wolf spiders (Araneae: Lycosidae). Anim Behav 52:167–181
Møller AP (1994) Sexual selection in the barn swallow (Hirundo rustica). IV. Patterns of fluctuating asymmetry and selection against asymmetry. Evolution 48:658–670
Møller AP (1998) Sexual selection and tail streamers in the barn swallow. Proc Roy Soc B 265:409–414
Page R, Ryan MJ (2008) The effect of signal complexity on localization performance in bats that localize frog calls. Anim Behav 76:761–769
Pröhl H, Hödl W (1999) Parental investment, potential reproductive rates and mating system in the strawberry poison-dart frog Dendrobates pumilio. Behav Ecol Sociobiol 46:215–220
Rand AS, Ryan MJ, Wilczynski W (1992) Signal redundancy and receiver permissiveness in acoustic mate recognition by the túngara frog, Physalaemus pustulosus. Am Zool 32:15–17
Ryan MJ (1985) The túngara frog: a study in sexual selection and communication. University of Chicago Press, Chicago
Ryan MJ, Rand AS (1990) The sensory basis of sexual selection for complex calls in the túngara frog, Physalaemus pustulosus (sexual selection for sensory exploitation). Evolution 44:305–314
Ryan MJ, Rand AS (2001) Feature weighting in signal recognition and discrimination by túngara frogs. In: Ryan MJ (ed) Anuran communication. Smithsonian Institution, Washington, D.C., pp 86–101
Ryan MJ, Rand AS (2003) Sexual selection in female perceptual space: how female túngara frogs perceive and respond to complex population variation in acoustic mating signals. Evolution 57:2608–2618
Ryan MJ, Tuttle MD, Rand AS (1982) Sexual advertisement and bat predation in a neotropical frog. Am Nat 119:136–139
Ryan MJ, Fox JH, Wilczynski W, Rand AS (1990) Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature 343:66–67
Ryan MJ, Rand W, Hurd PL, Phelps SM, Rand AS (2003) Generalization in response to mate recognition signals. Am Nat 161:380–394
Schwartz JJ, Gerhardt HC (1998) The neuroethology of frequency preferences in the spring peeper. Anim Behav 56:55–69
Sheldon BC, Andersson S, Griffith SC, Örnborg J, Sendecka J (1999) Ultraviolet colour variation influences blue tit sex ratios. Nature 402:874–877
Weigt LA, Crawford AJ, Rand AS, Ryan MJ (2005) Biogeography of the túngara frog, Physalaemus pustulosus. Mol Ecol 14:3857–3876
Zuk M, Thornhill R, Ligon JD, Johnson K, Austad S, Ligon SH, Thornhill NW, Costin C (1990) The role of male ornaments and courtship behavior in female mate choice of red jungle fowl. Am Nat 136:450–473
Zuk M, Lignon D, Thornhill R (1992) Effects of experimental manipulation of male secondary sex characters on female mate preference in red jungle fowl. Anim Behav 44:999–1006
Acknowledgments
We thank X. Bernal, A. Lea, L. Ziegler, A. Dapper, and N. Lessios for assistance with data collection and the staff at the Smithsonian Tropical Research Institute for logistical support. The suggestions of two anonymous reviewers significantly improved this manuscript. Autoridad Nacional del Ambiente approved scientific permits in the Republic of Panamá. Animal use was approved by the IACUC (#06041701) at The University of Texas at Austin. This work was supported by the National Science Foundation (grant number IOB 0544096 to M. Ryan). The Homer Lindsay Bruce Endowed Fellowship supported A. Baugh.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Christensen-Dalsgaard
Rights and permissions
About this article
Cite this article
Baugh, A.T., Ryan, M.J. The relative value of call embellishment in túngara frogs. Behav Ecol Sociobiol 65, 359–367 (2011). https://doi.org/10.1007/s00265-010-1053-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-1053-6