Abstract
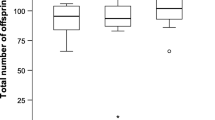
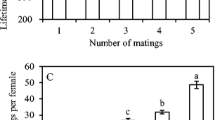
Species where most but not all females mate monandrously can provide insight into the potential factors both promoting and restricting polyandry. Polyandry is typically explained by direct and/or indirect benefits models; however, polyandry may also confer costs via sexually antagonistic processes. The fitness of polyandrous and monandrous females may also vary with environmental conditions, such as availability of water. For some lepidopterans, water is a vital resource that increases fecundity and may be a direct benefit of multiple mating. Male lepidopterans transfer large spermatophores that may be an important water source for females, particularly for species living in water-depauperate environments. In such species, multiple-mating females may increase their reproductive output. We examined the fitness consequences of multiple mating in the almond moth, Cadra cautella. Males transfer substantial spermatophores; these have a large chitinous process attached, which decrease female longevity. To assess the impact of female mating treatment and water availability on female fitness, females mated once or twice, either with the same or different males, with half the females in each treatment receiving water. Water-fed females had dramatically increased fecundity, but we found no fitness benefits of multiple mating. Male longevity decreased with increased mating frequency and potentially his level of reproductive investment. Water-deprived females that mated twice died sooner than once-mated females, while multiple-mating females that received water lived longer than their water-deprived counterparts. It is interesting to note that the male’s spermatophore process did not affect female fitness or longevity. Why polyandry is maintained in this species is discussed.



Similar content being viewed by others
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Arnqvist G, Rowe L (1995) Sexual conflict and arms races between the sexes—a morphological adaptation for control of mating in a female insect. Proc Royal Soc Biol Sci B 261:123–127
Arnqvist G, Rowe L (2005) Sexual conflict. Princeton University Press, Princeton, NJ
Arnqvist G, Edvardsson M, Friberg U, Nilsson T (2000) Sexual conflict promotes speciation in insects. Proc Natl Acad Sci USA 97:10460–10464
Boggs CL, Gilbert LE (1979) Male contribution to egg production in butterflies—evidence for transfer of nutrients at mating. Science 206:83–84
Bonduriansky R (2001) The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339
Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L (1995) Cost of mating in Drosophila melanogaster females is mediated by male accessory-gland products. Nature 373:241–244
Chow YS, Yen DF, Lin SH (1977) Water, a powerful attractant for gravid females of Plodia interpunctella and Cadra cautella. Experientia 33:453–455
Cook PA, Gage MJG (1995) Effects of risks of sperm competition on the numbers of eupyrene and apyrene sperm ejaculated by the moth Plodia interpunctella (Lepidoptera, Pyralidae). Behav Ecol Sociobiol 36:261–268
Crudgington HS, Siva-Jothy MT (2000) Genital damage, kicking and early death—the battle of the sexes takes a sinister turn in the bean weevil. Nature 407:855–856
Delisle J, Bouchard A (1995) Male larval nutrition in Choristoneura rosaceana (Lepidoptera, Tortricidae)—an important factor in reproductive success. Oecologia 104:508–517
Drummond BA (1984) Multiple mating and sperm competition in the Lepidoptera. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic, London, pp 291–370
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton, NJ
Edvardsson M (2007) Female Callosobruchus maculatus mate when they are thirsty: resource-rich ejaculates as mating effort in a beetle. Anim Behav 74:183–88
Ehrlich AH, Ehrlich PR (1978) Reproductive strategies in the butterflies: I. Mating frequency, plugging, and egg number. J Kans Entomol Soc 51:666–697
Forsberg J, Wiklund C (1989) Mating in the afternoon—time-saving in courtship and remating by females of a polyandrous butterfly Pieris napi. Behav Ecol Sociobiol 25:349–356
GenStat 7 Committee (2003) GenStat for Windows. Release 7.1. Lawes Agricultural Trust, Rothamsted, Oxford: VSN International
Greenfield MD (1982) The question of paternal investment in Lepidoptera—male-contributed proteins in Plodia interpunctella. Int J Invertebr Reprod 5:323–330
Ivy TM, Johnson JC, Sakaluk SK (1999) Hydration benefits to courtship feeding in crickets. Proc Royal Soc Biol Sci B 266:1523–1527
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev Camb Philos Soc 75:21–64
Karlsson B (1998) Nuptial gifts, resource budgets, and reproductive output in a polyandrous butterfly. Ecology 79:2931–2940
Kawagoe T, Suzuki N, Matsumoto K (2001) Multiple mating reduces longevity of females of the windmill butterfly Atrophaneura alcinous. Ecol Entomol 26:258–262
Mann T (1984) Spermatophores: development, structure, biochemical attributes and role in the transfer of spermatozoa. Springer, Berlin
Marcotte M, Delisle J, McNeil JN (2006) Impact of male mating history on the postmating resumption of sexual receptivity and lifetime reproductive success in Choristoneura rosaceana females. Physiol Entomol 31:227–233
McNamara KB, Jones TM, Elgar MA (2007) No cost of male mating experience on female reproductive success in the almond moth, Cadra cautella (Lepidoptera; Pyralidae). Behav Ecol Sociobiol 61:1177–1184
McNamara KB, Elgar MA, Jones TM (2008) Causes and consequences of variation in female mating frequency in the almond moth, Cadra cautella. Behaviour (in press)
Newcomer SD, Zeh JA, Zeh DW (1999) Genetic benefits enhance the reproductive success of polyandrous females. Proc Natl Acad Sci USA 96:10236–10241
Norris M (1932) Contributions towards the study of insect fertility I. The structure and the operation of the reproductive organs of the genera Ephestia and Plodia (Lepidoptera, Phycitidae). Proc Zool Soc Lond, pp 595–611
Norris M (1933) Contributions towards the study of insect fertility III. Adult nutrition, fecundity, and longevity in the genus Ephestia (Lepidoptera, Phycitidae). Proc Zool Soc Lond, pp 333–360
Oberhauser KS (1997) Fecundity, lifespan and egg mass in butterflies: effects of male-derived nutrients and female size. Funct Ecol 11:166–175
Pizzari T, Cornwallis CK, Lovlie H, Jakobsson S, Birkhead TR (2003) Sophisticated sperm allocation in male fowl. Nature 426:70–74
Rice WR (1996) Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381:232–234
Royer L, McNeil JN (1993) Male investment in the European corn-borer, Ostrinia nubilalis (Lepidoptera, Pyralidae)—impact on female longevity and reproductive performance. Funct Ecol 7:209–215
Ryne C, Ekeberg M, Olsson POC, Valeur PG, Lofstedt C (2002) Water revisited: a powerful attractant for certain stored-product moths. Entomol Exp Appl 103:99–103
Ryne C, Nilsson PA, Siva-Jothy MT (2004) Dietary glycerol and adult access to water: effects on fecundity and longevity in the almond moth. J Insect Physiol 50:429–434
SAS Institute (2000) JMP User’s Guide, Version 4.0.1. SAS Institute, Cary, North Carolina
Simmons LW (2001a) Sperm competition and its evolutionary consequences in the insects. Princeton University Press, Princeton, NJ
Simmons LW (2001b) The evolution of polyandry: an examination of the genetic incompatibility and good-sperm hypotheses. J Evol Biol 14:585–594
Torres-Vila LM, Stockel J, Rodriguez-Molina MC (1997) Physiological factors regulating polyandry in Lobesia botrana (Lepidoptera: Tortricidae). Physiol Entomol 22:387–393
Torres-Vila LM, Gragera J, Rodriguez-Molina MC, Stockel J (2002) Heritable variation for female remating in Lobesia botrana, a usually monandrous moth. Anim Behav 64:899–907
Torres-Vila LM, Rodriguez-Molina MC, Jennions MD (2004) Polyandry and fecundity in the Lepidoptera: can methodological and conceptual approaches bias outcomes? Behav Ecol Sociobiol 55:315–324
Tregenza T, Wedell N (2002) Polyandrous females avoid costs of inbreeding. Nature 415:71–73
Wedell N (1996) Mate quality affects reproductive effort in a paternally investing species. Am Nat 148:1075–1088
Wedell N, Cook PA (1999) Butterflies tailor their ejaculate in response to sperm competition risk and intensity. Proc Royal Soc Biol Sci B 266:1033–1039
Wedell N, Wiklund C, Cook PA (2002) Monandry and polyandry as alternative lifestyles in a butterfly. Behav Ecol 13:450–455
Wiklund C, Kaitala A, Lindfors V, Abenius J (1993) Polyandry and its effect on female reproduction in the green-veined white butterfly (Pieris napi L). Behav Ecol Sociobiol 33:25–33
Acknowledgements
We thank Alana Danne, Fiona Downs and Elizabeth Sim for their help in data collection and the Stored Grain Research Laboratory at the CSIRO for providing us with the moth culture. TMJ was funded by the Australian Research Council (DP0558265).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L .W. Simmons
Rights and permissions
About this article
Cite this article
McNamara, K.B., Elgar, M.A. & Jones, T.M. A longevity cost of re-mating but no benefits of polyandry in the almond moth, Cadra cautella . Behav Ecol Sociobiol 62, 1433–1440 (2008). https://doi.org/10.1007/s00265-008-0573-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0573-9