Abstract
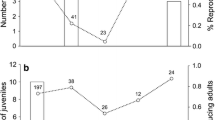
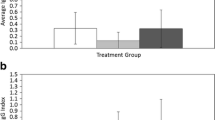
Parasitized animals may alter their life histories to minimize the costs of parasitism. Organisms are predicted to decrease investment in current reproduction when parasitism has the greatest impact on current reproductive ability. In contrast, if parasitism decreases residual reproductive value, hosts should increase current reproductive investment, referred to as fecundity compensation or terminal investment. In mammalian hosts, parasitic infection most often leads to reductions in current host reproduction, perhaps attributable to the emphasis on parasites that are unlikely to impact the host’s residual reproductive value. In this study, the life history response of a rodent, Peromyscus maniculatus, to infection with a parasite that should strongly impact the residual reproductive value of its host (Schistosomatium douthitti, Trematoda) was examined. Infection decreased survival for hosts exposed to a high dose of parasites and was chronic in survivors, confirming that infection had strong impacts for the residual reproductive value of the host. As predicted, infected mice increased their reproductive output, producing litters of greater mass due to heavier offspring. However, this increased output was observed after a greater delay to begin breeding in infected mice and was not observed in animals that suffered early mortality. The deer mouse S. douthitti system may provide a rare example of fecundity compensation in mammals.



Similar content being viewed by others
References
Adamo SA (1999) Evidence for adaptive changes in egg laying in crickets exposed to bacteria and parasites. Anim Behav 57:117–124
Agnew P, Koella JC, Michalakis Y (2000) Host life history responses to parasitism. Microbes and Infection 2:891–896
Allander K, Bennett GF (1995) Retardation of breeding inset in great tits (Parus major) by blood parasites. Func Ecol 9:677–682
Bindseil E, Hau J (1991) Negative effect on early postimplantation pregnancy and progesterone levels in mice infected with the intestinal trematode Echinostoma caproni. Parasitology 102:387–390
Bonneaud C, Mazuc J, Chastel O, Westerdahl H, Sorci G (2004) Terminal investment induced by immune challenge and fitness traits associate with major histocompatibility complex in the house sparrow. Evolution 58:2823–2830
Boonstra R, Krebs CJ, Beacham TD (1980) Impact of botfly parasitism on Microtus townsendii populations. Can J Zool 58:1683–1692
Botten J, Ricci R, Hjelle B (2001) Establishment of a deer mouse (Peromyscus maniculatus rufinus) breeding colony from wild-caught founders: comparison of reproductive performance of wild-caught and laboratory-reared pairs. Comp Med 51:314–318
Branson DH (2003) Effects of a parasitic mite on life-history variation in two grasshopper species. Evol Ecol Res 5:397–409
Burns CE, Goodwin BJ, Ostfeld RS (2005) A prescription for longer life? Bot fly parasitism of the white-footed mouse. Ecology 86:753–761
Chadwick W, Little TJ (2005) A parasite-mediated life-history shift in Daphnia magna. Proc R Soc Lond B Biol Sci 272:505–509
Clutton-Brock TH (1984) Reproductive effort and terminal investment in iteroparous animals. Am Nat 123:212–229
de Lope F, Moller AP (1993) Effects of ectoparasites on reproduction of their swallow hosts: a cost of being multi-brooded. Oikos 67:557–562
Derting TL, Virk MK (2005) Positive effects of testosterone and immunochallenge on energy allocation to reproductive organs. J Comp Physiol 175:543–556
Dewsbury DA (1979) Copulatory behavior of deer mice (Peromyscus maniculatus): II. A study of some factors regulating the fine structure of behavior. J Comp Physiol Psych 93:161–177
Fairbairn DJ (1977) The spring decline in deer mice: death or dispersal. Can J Zool 55:84–92
Fairbairn DJ (1978) Dispersal of deer mice, Peromyscus maniculatus: proximal causes and effects on fitness. Oecologia 32:171–193
Forbes MRL (1993) Parasitism and host reproductive effort. Oikos 67:444–450
Fryer SE, Oswald RC, Probert AJ, Runham NW (1990) The effect of Schistosoma haematobium infection on the growth and fecundity of three sympatric species of bulinid snails. J Parasitol 76:557–563
Fuller CA, Blaustein AR (1996) Effects of the parasite Eimeria arizonensis on survival of deer mice (Peromyscus maniculatus). Ecology 77:2196–2202
Gandon S, Agnew P, Michalakis Y (2002) Coevolution between parasite virulence and host life-history traits. Am Nat 160:374–388
Hammond KA, Kristan DM (2000) Responses to lactation and cold exposure by deer mice (Peromyscus maniculatus). Physiol Biochem Zool 73:547–556
Hayes JP (1989) Altitudinal and seasonal effects on aerobic metabolism of deer mice. J Comp Physiol 159:453–459
Hurd H (2001) Host fecundity reduction: a strategy for damage limitation. Trends Parasitol 17:363–368
Kagan IG, Meranze DR (1957) The histopathology of the liver in mice experimentally infected with Schistosomatium douthitti. J Infect Dis 100:23–29
Kalcounis-Rueppell MC, Millar JS, Herdman EJ (2002) Beating the odds: effects of weather on a short-season population of deer mice. Can J Zool 80:1594–1601
Kolluru GR, Zuk M, Chappell MA (2002) Reduced reproductive effort in male field crickets infested with parasitoid fly larvae. Behav Ecol 13:607–614
Kristan DM (2002) Maternal and direct effects of the intestinal nematode Heligmosomoides polygyrus on offspring growth and susceptibility to infection. J Exp Biol 205:3967–3977
Kristan DM (2004) Intestinal nematode infection affects host life history and offspring susceptibility to parasitism. J Anim Ecol 73:227–238
Malek EA (1977) Geographical distribution, hosts, and biology of Schistosomatium douthitti (Cort, 1924) Price, 1931. Can J Zool 55:661–671
McCurdy DG, Forbes MR, Boates JS (1999) Testing alternative hypotheses for variation in amphipod behaviour and life history in relation to parasitism. Int J Parasitol 29:1001–1009
McCurdy DG, Boates JS, Forbes MR (2001) An empirical model of the optimal timing of reproduction for female amphipods infected by trematodes. J Parasitol 87:24–30
Meagher S, O’Connor TP (2001) Population variation in the metabolic response of deer mice to infection with Capillaria hepatica (Nematoda). Can J Zool 79:554–561
Meikle D, Westberg M (2001a) Maternal nutrition and reproduction of daughters in wild house mice (Mus musculus). Reproduction 122:437–442
Meikle D, Westberg M (2001b) Social dominance rank and accessory glands in wild adult male house mice born to food-deprived mothers. Physiol Behav 72:359–364
Millar JS (1979) Energetics of lactation in Peromyscus maniculatus. Can J Zool 57:1015–1019
Millar JS (1983) Negative maternal effects in Peromyscus maniculatus. J Mammal 64:540–543
Millar JS (1985) Life cycle characteristics of Peromyscus maniculatus nebrascensis. Can J Zool 63:1280–1284
Millar JS, Innes DGL (1983) Demographics and life cycle characteristics of montane deer mice. Can J Zool 61:574–585
Millar JS, Derrickson EM, Sharpe STP (1992) Effects of reproduction on maternal survival and subsequent reproduction in northern Peromyscus maniculatus. Can J Zool 70:1129–1134
Minchella DJ (1985) Host life-history variation in response to parasitism. Parasitology 90:205–216
Minchella DJ, LoVerde PT (1981) A cost of increased early reproductive effort in the snail Biomphalaria glabrata. Am Nat 118:876–881
Neuhaus P (2003) Parasite removal and its impact on litter size and body condition in Columbian ground squirrels (Spermophilus columbianus). Proc R Soc Lond B Biol Sci (suppl.) 270:S213–S215
Norris K, Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol 11:19–26
Perrin N, Christe P, Richner H (1996) On host life-history response to parasitism. Oikos 75:317–320
Pianka ER, Parker WS (1975) Age-specific reproductive tactics. Am Nat 109:453–464
Polak M, Starmer WT (1998) Parasite-induced risk of mortality elevates reproductive effort in male Drosophila. Proc R Soc Lond B Biol Sci 265:2197–2201
Price HF (1931) Life History of Schistosomatium douthitti (Cort). Am J Hyg 13:685–727
Richner H (1998) Host-parasite interactions and life-history evolution. Zool-Anal Complex Syst 101:333–344
Sanz JJ, Arriero E, Moreno J, Merino S (2001) Interactions between hemoparasite status and female age in the primary reproductive output of pied flycatchers. Oecologia 126:339–344
Schlichting CD, Pigliucci M (1998) Phenotypic evolution: a reaction norm perspective. Sinauer, Sunderland
Schwanz LE (2006a) Reproductive investment when condition varies: optimality models and an experiment with deer mice (Peromyscus maniculatus). Ph.D. Dissertation, University of New Mexico
Schwanz LE (2006b) Schistosome infection in deer mice (Peromyscus maniculatus): impacts on host physiology, behavior and energetics. J Exp Biol 209:5029–5037
Schwanz LE (2008) Persistent effects of maternal parasitic infection on offspring fitness: implications for adaptive reproductive strategies when parasitized. Func Ecol (in press)
Sheldon BC, Verhulst S (1996) Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Sorci G, Clobert J, Michalakis Y (1996) Cost of reproduction and cost of parasitism in the common lizard, Lacerta vivipara. Oikos 76:121–130
Stearns SC (1992) The Evolution of Life Histories. Oxford University Press, New York
Telfer S, Bennett M, Bown K, Carslake D, Cavanagh R, Hazel S, Jones T (2005) Infection with cowpox virus decreases female maturation rates in wild populations of woodland rodents. Oikos 109:317–322
Thornhill JA, Jones JT, Kusel JR (1986) Increased oviposition and growth in immature Biomphalaria glabrata after exposure to Schistosoma mansoni. Parasitology 93:443–450
Weil ZM, Martin LB, Workman JL, Nelson RJ (2006) Immune challenge retards seasonal reproductive regression in rodents: evidence for terminal investment. Biol Lett 2:393–396
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100:687–690
Willis C, Poulin R (1999) Effects of the tapeworm Hymenolepis diminuta on maternal investment in rats. Can J Zool 77:1001–1005
Zajac AM, Williams JF (1981) The pathology of infection with Schistosomatium douthitti in the laboratory mouse and the meadow vole, Microtus pennsylvanicus. J Comp Physiol 91:1–10
Zar JH (1999) Biostatistical Analysis, 4th edn. Prentice Hall, New Jersey
Zuk M (1987) The effects of gregarine parasites on longevity, weight loss, fecundity and developmental time in the field crickets Gryllus veletis and Gryllus pennsylvanicus. Ecol Entomol 12:349–354
Zuk M, Stoehr AM (2002) Immune defense and host life history. Am Nat 160:S9–S22
Acknowledgements
Deer mice were provided by B. Hjelle (UNM MTA). Snails and S. douthitti were provided courtesy of D. Daniell (Butler University). L. Hertel and E. S. Loker kindly provided materials and training for parasitology techniques, and R. Ricci and F. Gurule provided all animal care. I thank H. Lease, E. Schultz, D. Swenton, C. Tech, and R. Ricci for assistance with data collection. Many people provided advice and assistance on the design and implementation of this study, discussion of the results, and improvements to this manuscript including J. Bragg, J. Brown, E. Charnov, D. Daniell, M. Festa-Bianchet, A. Kodric-Brown, D. Kristan, L. Hertel, E. Loker, R. Ricci, members of the Kodric-Brown and Loker Labs, and several anonymous reviewers. Funding was provided by a National Science Foundation Graduate Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Heeb
Rights and permissions
About this article
Cite this article
Schwanz, L.E. Chronic parasitic infection alters reproductive output in deer mice. Behav Ecol Sociobiol 62, 1351–1358 (2008). https://doi.org/10.1007/s00265-008-0563-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0563-y