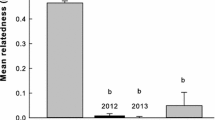
Abstract
Common shrews (Sorex araneus) maintain a foraging territory for most of their immature life. Possessing a high-quality territory is vital for overwinter survival in the harsh boreal climate, and hence, competitive ability in territorial disputes is expected to be an important component of individual fitness. To test possible association between individual inbreeding and fitness, we used neutral arena trials to assess the competitive performance of young common shrews. The experiment involved pairs of individuals originating from small island populations, where breeding must often occur between related individuals, and from large outbred mainland populations. The percentage of neutral arena tests that an individual won was highly significantly explained by internal relatedness, a surrogate measure of individual inbreeding, measured using ten microsatellite markers. Body size, sex, learning, and population type (mainland vs island) made no significant contributions. Even a low level of individual inbreeding may lead to significant adverse consequences in multiple territorial contests, which may represent a significant cause of inbreeding depression in many wild vertebrate populations.



Similar content being viewed by others
References
Allen DS, Aspey WP (1986) Determinants of social dominance in eastern gray squirrels (Sciurus carolinensis): a quantitative assessment. Anim Behav 34:81–89
Amos W, Wilmer JW, Fullard K, Burg TM, Croxall JP, Bloch D, Coulson T (2001) The influence of parental relatedness on reproductive success. Proc R Soc Lond B Biol Sci 268:2021–2027
Balloux F, Ecoffey E, Fumagalli L, Goudet J, Wyttenbach A, Hausser J (1998) Microsatellite conservation, polymorphism, and GC content in shrew of the genus Sorex (Insectivora, mammalia). Mol Biol Evol 15:473–475
Balloux F, Brunner H, Lugon-Moulin N, Hausser J, Goudet J (2000) Microsatellites can be misleading: an empirical and simulation study. Evolution 54:1414–1422
Balloux F, Amos W, Coulson T (2004) Does heterozygosity estimate inbreeding in real populations? Mol Ecol 13:3021–3032
Barnard CJ, Brown CAJ (1982) The effects of prior residence, competitive ability and food availability on the outcome of interactions between shrews (Sorex araneus L.). Behav Ecol Sociobiol 10:307–312
Beauchamp G (2004) Reduced flocking by birds on islands with relaxed predation. Proc R Soc Lond B Biol Sci 271:1039–1042
Bierne N, Launey S, Naciri-Graven Y, Bonhomme F (1998) Early effects of inbreeding as revealed by microsatellite analysed on Ostrea edulis larvae. Genetics 148:1893–1906
Brown JH, Ross B, McCauley S, Dance S, Taylor AC, Huntingford FA (2002) Resting metabolic rate and social status in juvenile giant freshwater prawns Macrobrachium rosenbergi. Mar Freshw Behav Physiol 36:31–40
Charlesworth D (1991) The apparent selection on neutral marker loci in partially in breeding populations. Genet. Res. 57:159–175
Chase ID, Bartolomeo C, Dugatkin LA (1994) Aggressive interactions and inter-contest interval: how long do winners keep winning? Anim Behav 48:393–400
Churchfield S (1990) Natural history of shrews. Christopher Helm, London
Clutton-Brock TH (1988) Reproductive success. The University of Chicago Press, Chicago
Crnokrak P, Roff AD (1999) Inbreeding depression in the wild. Heredity 83:260–270
Croin-Michielsen N (1966) Intraspecific and interspecific competition in the shrews Sorex araneus L. and Sorex minutus L. Arch Neerl Zool 17:73–174
Crowfort P (1957) The life of the shrew. Max Reinhardt, London
David P, Delay B, Berthou P, Jarne P (1995) Alternative models for allozyme-associated heterosis in the marine bivalve Spisula ovalis. Genetics 139:1719–1726
Dieringer D, Schlötterer C (2002) Microsatellite analyser (MSA): a platform independent analysis tool for large microsatellite data sets. Mol Ecol Notes 3:167–169
Eklund A (1996) The effects of inbreeding on aggression in wild house mice (Mus domesticus). Behaviour 133:883–901
Eldridge MDB, King JM, Loupis AK, Spencer PBS, Taylor AC, Pope LC, Hall GP (1999) Unprecedented low levels of genetic variation and inbreeding depression in an island population of the black-footed rock-wallaby. Conserv Biol 13:531–541
Falconer DS (1981) An introduction to quantitative genetics. Longman, London
Foster JB (1964) Evolution of mammals on islands. Nature 202:234–235
Frankham R (1997) Do island populations have less genetic variation than mainland populations? Heredity 78:311–318
Frankham R (1998) Inbreeding and extinction: island populations. Conserv Biol 12:665–675
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140
Guo SW, Thompson EA (1992) Performing the exact test of Hardy–Weinberg proportion for multiple alleles. Biometrics 48:359
Hanski I (1984) What does a shrew do in an energy crisis? In: Smith RH, Sibly RM (eds) Behaviour ecology. Blackwell, Oxford
Hanski I (1993) Dynamics of small mammals on islands. Ecography 16:372–375
Hanski I, Kuitunen J (1986) Shrews on small islands: epigenetic variation elucidates population stability. Holarct Ecol 9:193–204
Hanski I, Peltonen A, Kaski L (1991) Natal dispersal and social dominance in the common shrew. Oikos 62:48–57
Hedrick PW, Kalinowski ST (2000) Inbreeding depression in conservation biology. Ann Rev Ecol Syst 31:139–162
Hildner KK, Soulé ME (2004) Relationship between the energetic cost of burrowing and genetic variability among populations of the pocket gopher, T. bottae: does physiological fitness correlate with genetic variability? J Exp Biol 207:2221–2227
Hoffman JI, Boyd IL, Amos W (2004) Exploring the relationship between parental relatedness and male reproductive success in the Antarctic fur seal (Arctocephalus gazella). Evolution 58:2087–2099
Holand Ø, Gjøstein H, Losvar A, Kumpula J, Smith ME, Røed KH, Nieminen M, Weladji RB (2004) Social rank in female reindeer (Rangifer tarandus): effects of body mass, antler size and age. J Zool 263:365–372
Hsu Y, Wolff LL (1999) The winner and loser effect: integrating multiple experiences. Anim Behav 57:903–910
Huntingford FA, Turner A (1987) Animal conflict. Chapman & Hall, London
Kawano K (1992) Aggressive behavior of the domesticated house musk shrew (Suncus murinus) in inter-male inter-female and heterosexed interactions. J Ethol 10:119–132
Lahti K, Huuskonen H, Laurila A, Piironen J (2002) Metabolic rate and aggressiveness between brown trout populations. Funct Ecol 16:167–174
Madsen T, Stille B, Shine R (1996) Inbreeding depression in an isolated population of adders (Vipera berus). Biol Conserv 75:113–118
Magnanou E, Fons R, Blondel J, Morand S (2005) Energy expenditure in Crocidurinae shrews (Insectivora): is metabolism a key component of the insular syndrome? Comp Biochem Physiol A 142:276–285
Meagher S, Penn DJ, Potts WK (2000) Male–male competition magnifies inbreeding depression in wild house mice. Proc Natl Acad Sci USA 97:3324–3329
Metcalfe NB, Taylor AC, Thorpe JE (1995) Metabolic rate, social status and life-history strategies in Atlantic salmon. Anim Behav 49:431–436
Moraleva NV (1989) Intraspecific interactions in the common shrew Sorex araneus in Central Siberia. Ann Zool Fenn 26:425–432
Nevison CM, Barnard CJ, Hurst JL (2003) The consequence of inbreeding for modulating social relationships between competitors. Appl Anim Behav Sci 81:387–398
Queller DC, Goodnight KF (1989) Estimating relatedness using genetic markers. Evolution 43:258–275
Ralls K, Ballou J (1983) Extinction: lessons from zoos. In: Schonewald-Cox CM, Chambers SM, MacBryde B, Thomas L (eds) Genetics and conservation: a reference for managing wild animal and plant populations. Benjamin Cummings, Menlo Park, CA
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenism. J Heredity 86:248–249
Rutte C, Taborsky M, Brinkhof MWG (2006) What sets the odds of winning and loosing? TREE 21:16–21
Rychlik L (1998) Evolution of social systems in shrews. In: Wójcik JM, Wolsan M (eds) Evolution of shrews. Mammal Research Institute, Polish Academy of Sciences, Bialowieza
Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I (1998) Inbreeding and extinction in a butterfly metapopulation. Nature 392:491–494
Schuett GW (1997) Body size and agonistic experience affect dominance and mating success in male copperheads. Anim Behav 54:213–224
Slate J, David P, Dodds KG, Veenvliet BA, Glass BC, Broad TE, McEwan JC (2004) Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: theoretical expectations and empirical data. Heredity 93:255–265
Tiira K, Laurila A, Peuhkuri N, Piironen J, Ranta E, Primmer RP (2003) Aggressiveness is associated with genetic diversity in landlocked salmon. Mol Ecol 12:2399–2407
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Whitehouse MA (1997) Experience influences male–male contests in the spider Argyrodes antipodiana (Theridiidae: Araneae). Anim Behav 53:913–923
Wójcik JM, Borodin PM, Fedyk S, Fredga K, Hausser J, Mishta A, Orlov VN, Searle JB, Volobouev V, Zima J (2003) The list of the chromosome races of the common shrew Sorex araneus (updated 2002). Mammalia 67:169–178
Wyttenbach A, Favre L, Hausser J (1997) Isolation and characterization of simple sequence repeats in the genome of the common shrew. Mol Ecol 6:797–800
Zuri I, Rado R (2000) Sociality and agonistic behaviour in the lesser white-toothed shrew Crocidura suaveolens. J Mammal 81:606–616
Zwolak R, Rychlik L (2004) Does the reduction of locomotor activity serve as an aggression avoidance mechanism in shrews (Soricidae)? Electron J Pol Agric Univ Biol 7(2)
Acknowledgement
We thank Tomas Roslin and Sami Aikio for help with the analyses. Itsuro Koizumi, Luisa Orsini, Ilik Saccheri, and two anonymous referees made helpful comments on the manuscript. Anna Tuhti helped in the field work. This study was funded by the Academy of Finland (grant numbers 38604 and 44887, Finnish Centre of Excellence Programme, 2000–2005). The experimental procedures used were approved by the Experimental Animal Ethics Committee of the University of Helsinki, Finland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Trillmich
Rights and permissions
About this article
Cite this article
Välimäki, K., Hinten, G. & Hanski, I. Inbreeding and competitive ability in the common shrew (Sorex araneus). Behav Ecol Sociobiol 61, 997–1005 (2007). https://doi.org/10.1007/s00265-006-0332-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-006-0332-8