Abstract
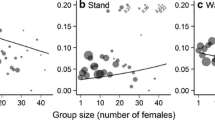
Rather than seeking females directly, males in many animal species locate and defend sites that contain spatially limited resources essential for female survival and reproduction. In these cases, resident males successfully repelling conspecific rivals will mate with sexually receptive females that seek to use the resident’s resources. Theory predicts that if resources are limiting in nature, are costly to procure, and if residency at the resource site increases male reproductive success, each site should be monopolised by a single adult male. Moreover, if females aggregate at these sites, males should be sedentary and monopolise harems. This predicts that males should reside at resource sites longer than females and male tenure should be positively correlated with harem size. I address these hypotheses using a wild population of the Wellington tree weta, Hemideina crassidens, a sexually dimorphic insect in which males use mandibular weapons in fights to control resource sites (galleries in trees) required by females for diurnal refuge. In a longitudinal study using artificial galleries, I show that male tenure in a gallery is positively related to harem size and, contrary to prediction, not gallery size per se. Moreover, contrary to prediction, females are more sedentary than males: males appear to move frequently between galleries to search for novel mates. I show experimentally that galleries represent mating sites to males: males prefer galleries housing adult females whereas females prefer unoccupied galleries. Females likely avoid male-occupied galleries because they incur injuries when interacting with males.



Similar content being viewed by others
References
Alcock J (1994) Postinsemination associations between males and females in insects: the mate-guarding hypothesis. Annu Rev Entomol 39:1–21
Bennington CC, Thayne WV (1994) Use and misuse of mixed model analysis of variance in ecological studies. Ecology 75:717–722
Bradbury JW, Vehrencamp SL (1977) Social organization and foraging in emballonurid bats III. Mating systems. Behav Ecol Sociobiol 2:1–17
Brown WD, Crespi BJ, Choe JC (1997) Sexual conflict and the evolution of mating systems. In: Choe JC, Crespi BJ (eds) The evolution of mating systems in insects and arachnids. Cambridge Univ Press, Cambridge, UK, pp 130–145
Darwin C (1874) The descent of man, and selection in relation to sex. J. Murray, London
Davies NB (1991) Mating systems. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach, 3rd edn. Blackwell Scientific Publications, Oxford, pp 263–294
Dodson GN (1997) Resource defense mating system in antlered flies, Phytalmia spp (Diptera: Tephritidae). Ann Entomol Soc Am 90:496–504
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Field LH (2001) Aggression behaviour in New Zealand tree wetas. In: Field LH (ed) The biology of wetas, king crickets and their allies. CAB International, Wallingford, pp 333–349
Field LH, Sandlant GR (1983) Aggression and mating behavior in the Stenopelmatidae (Orthoptera, Ensifera), with reference to New Zealand wetas. In: Gwynne DT, Morris GK (eds) Orthopteran mating systems-sexual competition in a diverse group of insects. Westview Press, Boulder, CO, pp 120–146
Field LH, Deans NA (2001a) Sexual selection and secondary sexual characters of wetas and king crickets. In: Field LH (ed) The biology of wetas, king crickets and their allies. CAB International, Wallingford, pp 179–204
Field LH, Jarman TH (2001b) Mating behaviour. In: Field LH (ed) The biology of wetas, king crickets and their allies. CAB International, Wallingford, pp 317–332
Field LH, Sandlant GR (2001c) The gallery-related ecology of New Zealand tree wetas, Hemideina femorata and Hemideina crassidens (Orthoptera, Anostostomatidae). In: Field LH (ed) The biology of wetas, king crickets and their allies. CAB International, Wallingford, pp 243–257
Fincke OM (1992) Consequences of larval ecology for territoriality and reproductive success of a neotropical damselfly. Ecology 73:449–462
Fincke OM, Hadrys H (2001) Unpredictable offspring survivorship in the damselfly, Megaloprepus coerulatus, shapes parental behavior, constrains sexual selection, and challenges traditional fitness estimates. Evolution 55:762–772
Fincke OM, Waage JK, Koenig WD (1997) Natural and sexual selection components of odonate mating patterns. In: Choe JC, Crespi BJ (eds) The evolution of mating systems in insects and arachnids. Cambridge Univ Press, Cambridge, UK, pp 58–74
Gibbs GW (2001) Habitats and biogeography of New Zealand’s Deinacridine and tusked weta species. In: Field LH (ed) The biology of wetas, king crickets and their allies. CAB International, Wallingford, pp 35–55
Greenfield MD (1997) Sexual selection in resource defense polygyny: lessons from territorial grasshoppers. In: Choe JC, Crespi BJ (eds) The evolution of mating systems in insects and arachnids. Cambridge Univ Press, Cambridge, UK, pp 75–88
Gwynne DT, Jamieson I (1998) Sexual selection and sexual dimorphism in a harem-polygynous insect, the alpine weta (Hemideina maori, Orthoptera Stenopelmatidae). Ethol Ecol Evol 10:393–402
Hudson GV (1892) Manual of New Zealand entomology. West, Newman, London
Hudson GV (1920) On some examples of New Zealand insects illustrating the Darwinian principle of sexual selection. Transactions and Proceedings of the New Zealand Institute 52:431–438
Jamieson IG, Forbes MR, McKnight EB (2000) Mark-recapture study of mountain stone weta Hemideina maori (Orthoptera: Anostostomatidae) on rock tor ‘islands’. NZ J Ecol 24:209–214
Kelly CD (2005) Allometry and sexual selection of male weaponry in Wellington tree weta, Hemideina crassidens. Behav Ecol 16:145–152
Kelly CD (2006) The relationship between resource control, association with females and male weapon size in a male-dominance insect. Ethology DOI 10.1111/j.1439.0310.2006.01193.x
Kirkendall LR, Kent DS, Raffa KF (1997) Interactions among males, females and offspring in bark and ambrosia beetles: the significance of living tunnels for the evolution of social behaviour. In: Choe JC, Crespi BJ (eds) The evolution of social behavior in insects and arachnids. Cambridge Univ Press, Cambridge, UK, pp 181–215
Mitchell PL (1980) Combat and territorial defense of Acanthocephala femorata (Hemiptera: Coreidae). Ann Entomol Soc Am 73:404–408
Reid ML, Roitberg BD (1994) Benefits of prolonged male residence with mates and brood in pine engravers (Coleoptera: Scolytidae). Oikos 70:140–148
Shuster SM, Wade MJ (2003) Mating systems and strategies. Princeton Univ Press, Princeton, NJ
Simmons LW (2001) Sperm competition and its evolutionary consequences in the insects. Princeton Univ Press, Princeton, NJ
Spencer AM (1995) Sexual maturity in the male tree weta Hemideina crassidens (Orthoptera: Stenopelamatidae). In: Zoology. Victoria University of Wellington, Wellington, New Zealand, pp 84
Stamps JA (1994) Territorial behavior: testing the assumptions. Adv Study Behav 23:173–232
Stringer IAN, Cary PRL (2001) Postembryonic development and related changes. In: Field LH (ed) The biology of wetas, king crickets and their allies. CAB International, Wallingford, pp 399–426
Switzer PV (1993) Site fidelity in predictable and unpredictable habitats. Evol Ecol 7:533–555
Switzer PV (1997) Factors affecting site fidelity in a territorial animal, Perithemis tenera. Anim Behav 53:865–877
Switzer PV (2002) Territory quality, habitat selection, and competition in the amberwing dragonfly, Perithemis tenera (Say) (Odonata: Libellulidae): Population patterns as a consequence of individual behavior. J Kans Entomol Soc 75:145–157
Trewick SA, Morgan-Richards M (2000) Artificial weta roosts: A technique for ecological study and population monitoring of Tree Weta (Hemideina) and other invertebrates. New Zealand Journal of Ecology 24:201–208
Tsubaki Y, Ono T (1986) Competition for territorial sites and alternative mating tactics in the dragonfly, Nannophya pygmaea Rambur (Odonata: Libellulidae). Behaviour 97:234–252
Wade MJ (1995) The ecology of sexual selection: Mean crowding of females and resource-defense polygyny. Evol Ecol 9:118–124
Wells KD (1977) Territoriality and male mating success in the green frog (Rana clamitans). Ecology 58:750–762
Wilkinson GS, Dodson GN (1997) Function and evolution of antlers and eye stalks in flies. In: Choe JC, Crespi BJ (eds) The evolution of mating systems in insects and arachnids. Cambridge Univ Press, Cambridge, UK, pp 310–328
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River, NJ
Acknowledgements
I thank Darryl Gwynne, Maydianne Andrade and three anonymous referees for the comments on the manuscript. I also thank George Gibbs for the valuable discussion and insight as well as Steve Ward of the New Zealand Department of Conservation for the logistical support and discussion whilst this work was conducted on Maud Island. This study was supported by grants from NSERC (Canada) and National Geographic Society to Darryl Gwynne.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Siva-Jothy
Rights and permissions
About this article
Cite this article
Kelly, C.D. Resource quality or harem size: what influences male tenure at refuge sites in tree weta (Orthoptera: Anostostomatidae)?. Behav Ecol Sociobiol 60, 175–183 (2006). https://doi.org/10.1007/s00265-005-0154-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0154-0