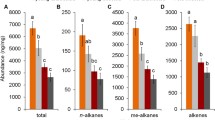
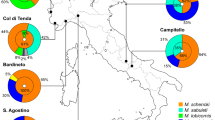
Abstract
Social parasites exploit the behaviours of other social species. Infiltration of host systems involves a variety of mechanisms depending on the conditions within the host society and the needs of the social parasite. For many species of socially parasitic ants, colony establishment entails the usurpation of colonies of other species. This frequently involves the eviction or death of the host colony queen and the subsequent adoption of the invading queen. The social parasite queen achieves host worker acceptance by either manipulating the nest-mate recognition processes of the host or undergoing chemical modification. Little is known, however, about how host workers respond to social parasite eggs or whether host species defend against brood parasitism during parasite invasions. Host species are believed to adopt social parasite offspring because the recent common ancestry between many social parasites and their hosts may grant the sharing of certain characteristics such as chemical cues. Use of multiple host species, however, suggests other processes are needed for the social bond between host and parasite young to form. This study reports the findings of adoption bioassays in which eggs from a slave-maker ant, Polyergus breviceps, were offered to workers of two of its host species from unparasitised or newly parasitised nests to determine whether P. breviceps eggs generally elicit rearing behaviours from multiple host species. Comparisons of parasite egg survival until adulthood with conspecific egg survival reveal that workers of both host species, free-living or newly enslaved, do not typically accept slave-maker eggs. Both host species thus have sufficient discriminatory power to reject social parasite eggs although our hydrocarbon analysis indicates parasite eggs may be adapted to their local host species. Combined these results suggest that host rearing of P. breviceps eggs may reflect an evolutionary equilibrium that is maintained by probability and cost of recognition errors.




Similar content being viewed by others
References
Agosti D (1994) A new inquiline ant (Hymenoptera: Formicidae) in Cataglyphis and its phylogenetic relationship. J Nat Hist 28:913–919
Akino T, Knapp JJ, Thomas JA, Elmes GW (1999) Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc R Soc Lond B 266:1419–1426
Alloway TM (1982) How the slave-making ant Harpagoxenus americanus (Emery) affects the pupa-acceptance behavior of its slaves. In: Breed MD, Michener CD, Evans HE (eds) The biology of social insects. Westview, Boulder, Colo., pp 261–265
Ayasse M, Paxton RJ (2002) Brood protection in social insects. In: Hilker M, Meiners T (eds) Chemoecology of insect eggs and egg deposition. Blackwell, Berlin, pp 117–148
Bagnères AG, Lorenzi MC, Dusticier G, Turillazzi S, Clement JL (1996) Chemical usurpation of a nest by paper wasp parasites. Science 272:889–892
Bhatkar A, Whitcomb WH (1970) Artificial diet for rearing various species of ants. Fla Entomol 53:229–232
Bourke AFG (1994) Indiscriminate egg cannibalism and reproductive skew in a multiple-queen ant. Proc R Soc Lond 255:55–59
Bourke AFG, Franks NR (1991) Alternative adaptations, sympatric speciation and the evolution of parasitic, inquiline ants. Biol J Linnean Soc 43:157–178
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton University Press, Princeton, N.J.
Breed MD, Snyder LE, Lynn TL, Morhart JA (1992) Acquired chemical camouflage in a tropical ant. Anim Behav 44:519–523
Cox DR (1972) Regression models and life-tables with discussion. J R Stat Soc B 34:187–220
Crozier RH, Dix MW (1979) Analysis of two genetic models for the innate components of colony odor in social Hymenoptera. Behav Ecol Sociobiol 47:217–224
Davies NB, Brooke MdL (1989) An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J Anim Ecol 58:207–224
Davies NB, Bourke AFG, Brooke MdL (1989) Cuckoos and parasitic ants: interspecific brood parasitism as an evolutionary arms race. Trends Evol Ecol 4:274–278
Dawkins R (1982) The extended phenotype. Oxford University Press, Oxford
D’Ettorre P, Errard C (1998) Chemical disguise during colony founding in the dulotic ant Polyergus rufescens Latr. (Hymenoptera, Formicidae). Insect Soc Life 2:71–77
D’Ettorre P, Heinze J (2001) Sociobiology of slave-making ants. Acta Ethol 3:67–82
D’Ettorre P, Errard C, Ibarra F, Francke W, Hefetz A (2000) Sneak in or repel your enemy: Dufour’s gland repellent as a strategy for successful usurpation in the slave-maker Polyergus rufescens. Chemoecology 10:135–142
D’Ettorre P, Mondy N, Lenoir A, Errard C (2002) Blending in with the crowd: social parasites integrate into their host colonies using a flexible chemical signature. Proc R Soc Lond B 269:1911–1918
Elmes GW, Barr B, Thomas JA, Clarke RT (1999) Extreme host specificity by Microdon mutabilis (Diptera: Syrphidae), a social parasite of ants. Proc R Soc Lond B 266:447–453
Emery C (1909) Über den Ursprung der dulotischen, parasitischen und myrmekophilen Ameisen. Biol Zentralbl 29:352–362
Errard C, D’Ettorre P (1998) Camouflage chimique chez la reine de Polyergus rufescens lors de la fondation. Actes Colloq Insect Soc 11:137–144
Foitzik S, Herbers JM (2001) Colony structure of a slave-makig ant. II. Frequency of slave raids and impact on the host population. Evolution 55:316–323
Foitzik S, DeHeer CJ, Hunjan DN, Herbers JM (2001) Coevolution in host–parasite systems: behavioural strategies of slave-making ants and their hosts. Proc R Soc Lond 268:1139–1146
Franks N, Blum M, Smith RK, Allies AB (1990) Behavior and chemical disguise of the cuckoo ant Leptothorax kutterii in relation to its host Leptothorax acervorum. J Chem Ecol 16:1431–1444
Gause GF (1935) The struggle for existence. Williams and Wilkins, Baltimore
Gladstone DE (1981) Why there are no ant slave rebellions. Am Nat 117:779–781
Goodloe L, Topoff H (1987) Pupa acceptance by slaves of the socially-parasitic ant Polyergus (Hymenoptera: Formicidae). Psyche 94:293–302
Hannonen M, Sundström L (2003) Worker nepotism among polygynous ants. Nature 421:910
Howard RW, Stanley-Samuelson DW, Akre R (1990) Biosynthesis and chemical mimicry of cuticular hydrocarbons from the obligate predator, Microdon albicomatus Novak (Diptera: Syrphidae) and its ant prey, Myrmica incompleta Provancher (Hymenoptera: Formicidae). J Kans Entomol Soc 63:437–443
Hölldobler B, Wilson EO (1990) The ants. Belknap, Cambridge, Mass.
Isingrini M, Lenoir A, Jaisson P (1985) Preimaginal learning as a basis of colony–brood recognition in the ant Cataglyphis cursor. Proc Nat Acad Sci USA 82:8545–8547
Jaisson P (1987) The construction of fellowship between nestmates in social Hymenoptera. Experientia [Suppl] 54:313–331
Jaisson P, Fresneau D (1978) The sensitivity and responsiveness of ants to their cocoons in relation to age and methods of measurement. Anim Behav 26:1064–1071
Johnson CA, Vander Meer RK, Lavine B (2001) Changes in the cuticular hydrocarbon profile of the slave-maker ant queen, Polyergus breviceps Emery, after killing a Formica host queen (Hymenoptera: Formicidae). J Chem Ecol 27:1787–1804
Johnson CA, Topoff H, Vander Meer RK, Lavine B (2002) Host queen killing by a slave-maker ant queen: when is a host queen worth attacking? Anim Behav 64:807–815
Kaib M, Heinze J, Ortius D (1993) Cuticular hydrocarbon profiles in the slave-making ant Harpagoxenus sublaevis and its hosts. Naturwissenschaften 80:281–285
Lahav S, Soroker V, Hefetz A, Vander Meer RK (1999) Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86:246–249
Lawes MJ, Marthews TR (2003) When will rejection of parasite nestlings by hosts of nonevicting avian brood parasites be favored? A misimprinting-equilibrium model. Behav Ecol 14:757–770
Lenoir A, Fresneau D, Errard C, Hefetz A (1999) Individuality and colonial identity in ants: the emergence of the social representation concept. In: Detrain C, Deneubourg JL, Pasteels J (eds) Information processing in social insects. Birkhäuser, Basel, pp 219–237
Lenoir A, D’Ettorre P, Errard C (2001) Chemical ecology and social parasitism in ants. Annu Rev Entomol 46:573–599
Lorenzi M C, Filippone F (2000) Opportunistic discrimination of alien eggs by social wasps (Polistes biglumis, Hymenoptera Vespidae): a defense against social parasitism? Behav Ecol Sociobiol 48:402–406
Lotem A, Nakamura H, Zahavi A (1995) Constraints on egg discrimination and cuckoo-host co-evolution. Anim Behav 49:1185–1209
Monnin T, Peeters C (1997) Cannibalism of subordinates’ eggs in the monogynous queenless ant Dinoponera quadriceps. Naturwissenschaften 84:499–502
Mori A, D’Ettorre P, Le Moli F (1995) Host nest usurpation and colony foundation in the European amazon ant, Polyergus rufescens Latr. (Hymenoptera: Formicidae). Insectes Soc 42:279–286
Mori A, Grasso DA, Visicchio R, Le Moli F (2000a) Colony founding in Polyergus rufescens: the role of the Dufour’s gland. Insectes Soc 47:7–10
Mori A, Visicchio R, Sledge MF, Grasso DA, Le Moli F, Turillazzi S, Spencer S, Jones GR (2000b) Behavioral assays testing the appeasement allomone of Polyergus rufescens queens during host-colony usurpation. Ethol Ecol Evol 12:315–322
Oliveira PS, Hölldobler B (1991) Agonistic interactions and reproductive dominance in Pachycondyla obscuricornis. Psyche 98:215–225
Otto M (1999) Chemometrics. Wiley, New York
Peeters C, Monnin T, Malosse C (1999) Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc R Soc Lond B 266:1323–1327
Plateaux L (1985) Adoption expérimentale entre des formis de genres différents Myrmica laevinodis Nylanderi et Anergates atratulus Schenk. Insectes Soc 32:221–226
Ratnieks FLW (1995) Evidence for a queen-produced egg-marking pheromone and its use in worker policing in the honey bee. J Apic Res 34:31–37
Savolainen R, Vepsäläinen K (2003) Sympatric speciation through intraspecific social parasitism. Proc Natl Acad Sci USA 100:7169–7174
Schumann R, Buschinger A (1991) Selective acceptance of alien host species pupae by slaves of the dulotic ant, Harpagoxenus sublaevis (Hymenoptera, Formicidae, Myrmicinae). Ethology 88:154–162
Singer TL (1998) Roles of hydrocarbons in the recognition systems of insects. Am Zool 38:394–405
Sumner S, Aanen DK, Delabie J, Boomsma JJ (2004) The evolution of social parasitism in Acromyrmex leaf-cutting ants: a test of Emery’s rule. Insectes Soc 51:37–42
Thompson JN (1994) The coevolutionary process. The University of Chicago Press, Chicago
Topoff H, Cover S, Greenberg L, Goodloe L, Sherman P (1988) Colony founding by queens of the obligatory slave-making ant, Polyergus breviceps: the role of the Dufour’s gland. Ethology 78:209–218
Topoff H, Weickert T, Zimmerli EJ (1990) A comparative study of colony takeover between queens of facultative and obligatory slave-making ants (Hymenoptera: Formicidae). J Insect Behav 3:813–817
Vander Meer RK, Morel L (1998) Nestmate recognition in ants. In: Vander Meer RK, Breed M, Winston M, Espelie KE (eds) Pheromone communication in social insects. Westview, Boulder, Colo., pp 79–103
Vander Meer RK, Jouvenaz DP, Wojcik DP (1989) Chemical mimicry in a parasitoid (Hymenoptera: Eucharitidae) of fire ants (Hymenoptera: Formicidae). J Chem Ecol 15:2247–2261
Vienne C, Soroker V, Hefetz A (1995) Congruency of hydrocarbon patterns in heterospecific groups of ants: transfer and/or biosynthesis? Insectes Soc 42:267–277
Wagner D, Brown MJF, Broun P, Cuevas W, Moses LE, Chao DL, Gordon DM (1998) Task-related differences in the cuticular hydrocarbon composition of harvester ants, Pogonomyrmex barbatus. J Chem Ecol 24:2021–2037
Yamaoka R (1990) Chemical approach to understanding interactions among organisms. Physiol Ecol Jpn 27:31–52
Zimmerli EJ, Mori A (1993) The role of an attractive brood pheromone in the obligatory, slavemaking ant, Polyergus breviceps (Hymenoptera: Formicidae). J Insect Behav 6:761–770
Acknowledgements
Field research was conducted at the Southwestern Research Station (SWRS) of the American Museum of Natural History, Portal, Ariz. Behavioural tests were conducted at SWRS, Hunter College, New York, N.Y. and the American Museum of Natural History, New York, N.Y. The chemical analyses were carried out at the Medical and Veterinary Entomology Research Laboratory of the United States Department of Agriculture. We thank Michele Hosack for her assistance in the chemical laboratory, Erica Gallegos for her assistance with initial nest egg monitoring, James Carpenter for comments on an early version of the manuscript, and David Nash, Lotta Sundström, Alain Lenoir, two anonymous reviewers and Joan Herbers and her laboratory for comments on a later version of the manuscript. Parts of this research were aided by grants from the Animal Behavior Society, Sigma Xi, Southwestern Research Station Student Support Fund, Theodore Roosevelt Memorial Fund and by a fellowship from the Biopsychology subprogram at Hunter College to C.A. Johnson.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Sundström
Rights and permissions
About this article
Cite this article
Johnson, C.A., Topoff, H., Vander Meer, R.K. et al. Do these eggs smell funny to you?: an experimental study of egg discrimination by hosts of the social parasite Polyergus breviceps (Hymenoptera: Formicidae). Behav Ecol Sociobiol 57, 245–255 (2005). https://doi.org/10.1007/s00265-004-0851-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0851-0