Abstract
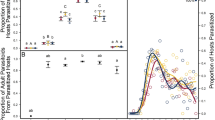
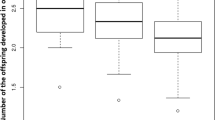
In insect parasitoids, offspring fitness is strongly influenced by the adult female’s choice of host, particularly in ectoparasitoids that attack non-growing host stages. We quantified the fitness consequences of size-dependent host species selection in Dirhinus giffardii, a solitary ectoparasitoid of tephritid fruit fly pupae. We first showed a positive correlation between the size of emerged D. giffardii wasps and the size of their host fruit fly species (in order of decreasing size): Bactrocera latifrons, B. cucurbitae, B. dorsalis or Ceratitis capitata. We then manipulated individual wasps to show that the parasitoid preferred to attack the largest (B. latifrons) to the smallest (C. capitata) host species when provided with a choice, and laid a greater proportion of female eggs in B. latifrons than in C. capitata. There were no differences in developmental time or offspring survival between individuals reared from these two host species. Finally, we compared the foraging efficiency of large versus small wasps (reared from B. latifrons vs C. capitata) under two different laboratory conditions: high versus low host habitat quality, given that realized fecundity in parasitoids may be influenced by either egg-limited or time-limited factors. Under both conditions, large wasps parasitized more hosts than did small ones as a consequence of high searching efficiency in the host-poor habitat, and high capacity for adjusting egg maturation in response to host availability in the host-rich habitat. Considering the flexibility of body growth, the apparent lack of cost of achieving large body size in either development or survival, and the strong dependence of realized reproductive success on a female’s size, we argue that body size may be a key to understanding evolution of host species selection in ectoparasitoids. We also discuss constraints upon the evolution of size-dependent host species selection in parasitoids.




Similar content being viewed by others
References
Assem J van den, van Iersel JJA, Los den Hartog RL (1989) Is being large more important for female than male parasitic wasps? Behavior 108:160–195
Bai GM (1990) Host-parasite relationship of Dirhinus pachycerus Masi (Hymenoptera: Chalcididae), with particular reference to its house fly control potential. In: Rutz DA, Patterson RS (eds) Biocontrol of arthropods affecting livestock and poultry. Westview, Boulder, Colo., pp 11–27
Bennett DM, Hoffmann AA (1998) Effects of size and fluctuating asymmetry on field fitness of the parasitoid Trichogramma carverae (Hymenoptera:Trichogrammatidae). J Anim Ecol 67:580–591
Charnov EL, Stephens DW (1988) On the evolution of host selection in solitary parasitoids. Am Nat 132:707–722
Charnov EL, Los den Hartog RL, Jones T, van den Assem J (1980) Sex ratio evolution in a variable environment. Nature 289:27–33
Clutton-Brock TH (1988) Reproductive success: studies of individual variations in contrasting breeding systems. University of Chicago Press, Chicago
Dresner E (1954) Observations on the biology and habits of pupal parasites of the oriental fruit fly. Proc Hawaii Entomol Soc 15:299–310
Eijs IEM, van Alphen JJM (1999) Life history correlations: why are hymenopteran parasitoids an exception? Ecol Lett 2:27–35
Ellers J, Jervis MA (2003) Body size and the timing of egg production in parasitoid wasps. Oikos 102:164–172
Ellers J, van Alphen JJM, Sevenster JG (1998) A field study of size-fitness relationship in the parasitoid Asobara tabida. J Anim Ecol 67:318–324
Fox CW, Czesak ME (2000) Evolutionary ecology of progeny size in arthropods. Annu Rev Entomol 45:341–369
Godfray HCJ (1994) Parasitoids, behavioural and evolutionary ecology. Princeton University Press, Princeton, N.J.
Harvey JA, Strand MR (2002) The developmental strategies of endoparasitoid wasps vary with host feeding ecology. Ecology 83:2439–2451
Harvey JA, Harvey IF, Thompson DJ (1994) Flexible larval growth allows use of a range of host sizes by a parasitoid wasp. Ecology 75:1420–1428
Harvey JA, Bezemer TM, Elzinga JA, Strand MR (2004) Development of the solitary endoparasitoid Microplitis demolitor: host quality does not increase with host age and size. Ecol Entomol 29:35–43
Heinz KM (1993) Sex-specific reproductive consequences of body size in the solitary ectoparasitoid Diglyphus begini. Evolution 45:1511–1515
Jervis MA, Ferns PN, Heimpel GE (2003) Body size and the timing of egg production in parasitoid wasps: a comparative analysis. Funct Ecol 17:375–383
Kazmer DJ, Luck RF (1995) Field tests of the size-fitness hypothesis in the egg parasitoid Trichogramma pretiosum. Ecology 76:412–425
King BH, Charnov EL (1988) Sex-ratio manipulation in response to host size by the parasitoid Spalangia cameroni: a laboratory study. Evolution 42:1190–1198
King BH, Lee HE (1994) Test of the adaptiveness of sex ratio manipulation in a parasitoid wasp. Behav Ecol Sociobiol 35:437–443
Klingenberg CP, Spence JR (1997) On the role of body size for life-history evolution. Ecol Entomol 22:55–68
Lafferty KD, Kuris AM (2002) Trophic strategies, animal diversity and body size. Trends Ecol Evol 17:507–513
Lapchin L (2002) Host-parasitoid association and diffuse coevolution: what to be a generalist? Am Nat 160:245–254
Leather SR (1988) Size, reproductive potential and fecundity in insects: things aren’t as simple as they seem. Oikos 51:386–388
Mackauer M, Sequeira R (1993) Patterns of development in insect parasites. In: Beckage NE, Thompson SN, Federici BA (eds) Parasites and pathogens of insects, V1. Academic, San Diego, pp 1–23
Morris RJ, Fellowes MDF (2002) Learning and natal host influence host preference, handling time and sex allocation behavior in a pupal parasitoid. Behav Ecol Sociobiol 51:386–393
Napoleon ME, King BH (1999) Offspring sex ratio response to host size in the parasitoid wasp Spalangia endius. Behav Ecol Sociobiol 46:325–332
Noyes JS (2002) Interactive catalogue of world Chalcidoidea (2001). Electronic compact disc by Taxapad, Vancouver Canada and the Natural History Museum, London
Otto M, Mackauer M (1998) The developmental strategy of an idiobiont ectoparasitoid, Dendrocerus carpenteri: influence of variations in host quality on offspring growth and fitness. Oecologia 117:353–364
Papaj DR (2000) Ovarian dynamics and host use. Annu Rev Entomol 45:423–448
Petersen G, Hardy IC (1996) The importance of being larger: parasitoid intruder-owner contests and their implications for clutch size. Anim Behav 51:1363–1373
Podoler H, Mazor M (1981) Dirhinus giffardii Silvestri (Hym.: Chalcididae) as a parasite of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Dip.: Tephritidae). I. Some biological studies. Acta Oecol 2:255–265
Quicke DJ (1997) Parasitic wasps. Chapman and Hall, London
Rivero A, West SA (2002) The physiological costs of being small in parasitic wasps. Evol Ecol Res 77:167–172
Roff DA, Mostowy S, Fairbairn DJ (2002) The evolution of trade-offs: testing predications on response to selection and environmental variation. Evolution 56:84–95
Roitberg BD, Boivin G, Vet LEM (2001) Fitness, parasitoids and biological control: an opinion. Can Entomol 133:429–438
Rolff J, Kraaijeveld AR (2001) Host preference and survival in selected lines of a Drosophila parasitoid, Asobara tabida. J Evol Biol 14:742–745
Rosenheim JA (1998) Higher-order predators and the regulation of insect herbivore populations. Annu Rev Entomol 43:421–447
Rosenheim JA (1999) The relative contributions of time and eggs to the cost of reproduction. Evolution 53:376–385
Sagarra LA, Vincent C, Stewart RK (2001) Body size as an indicator of parasitoid quality in male and female Anagyrus kamali (Hymenoptera: Encyrtidae). Bull Entomol Res 91:363–367
Sequeira R, Mackauer M (1992) Covariance of adult size and development time in the parasitoid wasp Aphidius ervi in relation to the size of its hosts, Acyrthosiphon pisum. Evol Ecol 6:34–44
Tammaru T, Esperk T, Castellanos I (2002) No evidence for costs of being large in females of Orgyia spp. (Lepidoptera, Lymantriidae): large is always better. Oecologia 133:430–438
Tanaka N, Steiner HF, Ohinata K, Okamota R (1969) Low-cost larvae rearing medium for mass production of oriental and Mediterranean fruit fly. J Econ Entomol 62:970–971
Teder T, Tammaru T, Pedmanson R (1999) Patterns of host use in solitary parasitoids (Hymenoptera, Ichneumonidae): field evidence from a homogeneous habitat. Ecography 22:79–86
Ueno T (1998a) Adaptiveness of sex ratio control by the pupal parasitoid Itoplectis naranyae (Hymenoptera: Ichneumonidae) in response to host size. Evol Ecol 12:643–654
Ueno T (1998b) Sex allocation by a parasitoid wasp (Hymenoptera: Ichneumonidae) to different host species: a question for the mechanism of host size. J Insect Behav 11:811–821
Visser MF (1994) The importance of being large: the relationship between size and fitness in females of the parasitoid Aphaereta minuta (Hymenoptera: Braconidae). J Anim Ecol 6:963–978
Wang XG, Messing RH (2003) Egg maturation in the parasitoid Fopius arisanus (Hymenoptera: Braconidae): do host-associated stimuli promote ovarian development? Ann Entomol Soc Am 96:571–578
Wang XG, Messing RH (2004a) The ectoparasitic pupal parasitoid, Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae), attacks other primary tephritid fruit fly parasitoids: host expansion and potential non-target impact. Biol Control (in press)
Wang XG, Messing RH (2004b) Potential interactions between pupal and egg- or larval-pupal parasitoids of tephritid fruit flies. Environ Entomol (in press)
West SA, Flanagan KE, Godfray HCJ (1996) The relationship between parasitoid size and fitness in the filed, a study of Achrysocharoides zwoelferi (Hymenoptera: Eulophidae). J Anim Ecol 65:631–639
Wharton RA (1989) Classical biological control of fruit Tephritidae. In: Robinson A, Harper G (eds) World crop pests, fruit flies: their biology, natural enemies, and control, V3b. Elsevier, Amsterdam, pp 303–313
Acknowledgements
We thank T. Moats for assistance, and M. Rhainds, M. Ramadan, A. Bokonon-Ganta, E. Jarjees and the three referees for their useful comments on earlier versions of the manuscript. We also thank the USDA-ARS Pacific Basin Agricultural Research Center, Honolulu, Hawaii for providing fruit flies, and E. Jarjess and M. Johnson for providing D. giffardii. This research was supported by USDA-ARS grant 5853208147 to R.H.M. This is publication no. 4685 of the University of Hawaii, College of Tropical Agriculture and Human Resources Journal Series.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Gwynne
Rights and permissions
About this article
Cite this article
Wang, X.G., Messing, R.H. Fitness consequences of body-size-dependent host species selection in a generalist ectoparasitoid. Behav Ecol Sociobiol 56, 513–522 (2004). https://doi.org/10.1007/s00265-004-0829-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0829-y