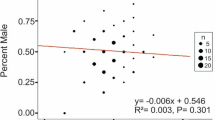
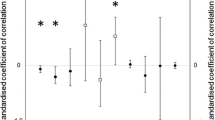
Abstract
A number of models have been proposed to provide adaptive explanations of sex-ratio variation in mammals. Two models have been applied commonly to primates and ungulates with varying success—the Trivers-Willard (TW) hypothesis, and the local resource competition (LRC) hypothesis. For polygynous, sexually dimorphic mammals, where males are larger and disperse more readily, these models predict opposite outcomes of sex-ratio adjustment within the same environmental context (high-resource years: TW—more sons; LRC—more daughters). However, many of the predictions of these two models can vary depending on factors influencing resource availability, such as environmental stochasticity, resource predictability, and population density. The New Zealand fur seal (Arctocephalus forsteri) is a polygynous mammal showing marked sexual dimorphism (larger males), with higher variation in male reproductive success expected. We provide clear evidence of male-biased sex ratios from a large sample of A. forsteri pups captured around South Island, New Zealand during 1996/1998, even after accounting for a sex bias in capture probability. The extent of the bias depended upon year and, in 1998, strong climatic perturbations (El Niño/Southern Oscillation, ENSO) probably reduced food availability. Significant male-biased sex ratios were found in all years; however, there was a significant decline in the male bias in 1998. There was no relationship between sex ratio and population density. We suggest that the sex-ratio bias resulted from the production of relatively more male pups. Under the density-independent scenario, the strong male bias in A. forsteri sex ratios is support for the TW model within an environment of high resource predictability. We suggest that some plasticity in the determination of pup sex among years is a mechanism by which A. forsteri females in New Zealand, and perhaps other otariid seals, can maximise fitness benefits when living in regions of high, yet apparently predictable, environmental variability. We also suggest that much of the inconsistency in the reported sex ratios for otariid seals results from the complex interaction of population density and environmental stochasticity influencing relative food availability over time.





Similar content being viewed by others
References
Antonelis GA, Ragen TJ, Rooks NJ (1994) Male-biased secondary sex ratios of northern fur seals on the Pribilof Islands, Alaska, 1989 and 1992. In: Sinclair EH (ed) Fur seal investigations, 1992. Technical Memo NMFS-AFSC-45. US Department of Commerce, National Oceanographic and Atmospheric Administration, Seattle, pp 84–89
Arnbom T, Fedak MA, Rothery P (1994) Offspring sex ratio in relation to female size in southern elephant seals, Mirounga leonina. Behav Ecol Sociobiol 35:373–378
Arnould JPY, Boyd IL, Rawlins DR, Hindell MA (2001) Variation in maternal provisioning by lactating Antarctic fur seals (Arctocephalus gazella): response to experimental manipulation in pup demand. Behav Ecol Sociobiol 50:461–466
Austad SN, Sunquist ME (1986) Sex-ratio manipulation in the common opossum. Nature 324:58–60
Bartholomew GA (1970) A model for the evolution of pinniped polygyny. Evolution 24:546–559
Basher RE (1998) The 1997/98 El Niño event: impacts, responses and outlook for New Zealand. 73. Ministry of Research, Science and Technology, Wellington
Boltnev AI, York AE, Antonelis GA (1998) Northern fur seal young: interrelationships among birth size, growth, and survival. Can J Zool 76:843–854
Bonner WN (1968) The fur seal of South Georgia. Rep. No. 56. British Antarctic Survey, London
Boyd IL (2000) State-dependent fertility in pinnipeds: contrasting capital and income breeders. Funct Ecol 14:623–630
Boyd IL, McCann TS (1989) Pre-natal investment in reproduction by female Antarctic fur seals. Behav Ecol Sociobiol 24:377–385
Boyd IL, Lunn NJ, Rothery P, Croxall JP (1995) Population demography of Antarctic fur seals: the costs of reproduction and implications for life-histories. J Anim Ecol 64:505–518
Boyd IL, McCafferty DJ, Reid K, Taylor R, Walker TR (1998) Dispersal of male and female Antarctic fur seals (Arctocephalus gazella). Can J Fish Aquat Sci 55:845–852
Bradshaw CJA, Lalas C, Perriman L, Harcourt RG, Best H, Davis LS (1999) Seasonal oscillation in shore attendance and transience of New Zealand fur seals. Can J Zool 77:814–823
Bradshaw CJA, Davis LS, Lalas C, Harcourt RG (2000a) Geographic and temporal variation in the condition of pups of the New Zealand fur seal (Arctocephalus forsteri): evidence for density dependence and differences in the marine environment. J Zool Lond 252:41–51
Bradshaw CJA, Lalas C, Thompson CM (2000b) Clustering of colonies in an expanding population of New Zealand fur seals (Arctocephalus forsteri). J Zool Lond 250:105–112
Bradshaw CJA, Davis LS, Purvis M, Zhou Q, Benwell GL (2002) Using artificial neural networks to model the suitability of coastline for breeding by New Zealand fur seals. Ecol Model 148:111–131
Bradshaw CJA, Barker RJ, Harcourt RG, Davis LS (2003) Estimating survival and capture probability of fur seal pups using multi-state mark-recapture models. J Mammal 84:65–80
Buckland ST, Burnham KP, Augustin NH (1997) Model selection: an integral part of inference. Biometrics 53:603–618
Buskirk SW, Lindstedt SL (1989) Sex biases in trapped samples of Mustelidae. J Mammal 70:88–97
Byholm P, Ranta E, Kaitala V, Lindén H, Saurola P, Wikman M (2002) Resource availability and goshawk offspring sex ratio variation: a large-scale ecological phenomenon. J Anim Ecol 71:994–1001
Calambokidis J, Gentry RL (1985) Mortality of northern fur seal pups in relation to growth and birth weights. J Wildl Dis 21:327–330
Cameron EZ, Linklater WL (2000) Individual mares bias investment in sons and daughters in relation to their condition. Anim Behav 60:359–367
Cameron EZ, Linklater WL, Stafford KJ, Veltman CJ (1999) Birth sex ratios relate to mare condition at conception in Kaimanawa horses. Behav Ecol 10:472–475
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Clark AB (1978) Sex ratio and local resource competition in a prosimian primate. Science 201:163–165
Clark MM, Galef BG Jr (1995) A gerbil dam's fetal intrauterine position affects the sex ratios of litters she gestates. Physiol Behav 57:297–299
Clutton-Brock TH (1982) Parental investment in male and female offspring in mammals. In: Group KCS (ed) Current problems in sociobiology. Cambridge University Press, Cambridge, pp 223–247
Clutton-Brock TH, Iason GR (1986) Sex ratio variation in mammals. Rev Biol 61:339–374
Clutton-Brock TH, Albon SD, Guinness FE (1981) Parental investment in male and female offspring in polygynous mammals. Nature 289:487–489
Clutton-Brock TH, Albon SD, Guinness FE (1984) Maternal dominance, breeding success and birth sex ratios in red deer. Nature 308:358–360
Cockburn A, Legge S, Double MC (2002) Sex ratios in birds and mammals: can the hypotheses be disentangled? In: Hardy ICW (ed) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge, pp 267–286
Costa DP, Trillmich F, Croxall JP (1988) Intraspecific allometry of neonatal size in the Antarctic fur seal (Arctocephalus galapagoensis). Behav Ecol Sociobiol 22:361–364
Côté SD, Festa-Bianchet M (2001) Offspring sex ratio in relation to maternal age and social rank in mountain goats (Oreamnos americanus). Behav Ecol Sociobiol 49:260–265
Crawley MC (1975) Growth of New Zealand fur seal pups. N Z J Mar Freshwater Res 9:539–545
Dickman CR (1988) Sex-ratio variation in response to interspecific competition. Am Nat 132:289–297
Dittus WPJ (1998) Birth sex ratios in toque macaques and other mammals: integrating the effects of maternal condition and competition. Behav Ecol Sociobiol 44:149–160
Doidge DW, Croxall JP, Baker JR (1984) Density-dependent pup mortality in the Antarctic fur seal Arctocephalus gazella at South Georgia. J Zool Lond 202:449–460
Fisher RA (1930) The genetical theory of natural selection. Oxford University Press, Oxford
Fowler CW (1990) Density dependence in northern fur seals (Callorhinus ursinus). Mar Mammal Sci 6:171–195
Francis JM, Heath CB (1991) The effects of El Niño on the frequency and sex ratio of suckling yearlings in the California sea lion. In: Trillmich F, Ono KA (eds) Pinnipeds and El Niño: responses to environmental stress, vol 88. Springer, Berlin Heidelberg New York, pp 193–201
Frank SA (1990) Sex allocation theory for birds and mammals. Annu Rev Ecol Syst 21:13–55
Gehrt SD, Fritzell EK (1996) Sex-biased response of raccoons (Procyon lotor) to live traps. Am Midl Nat 135:23–32
Gentry RL, Kooyman GL (1986) Fur seals: maternal strategies on land and at sea. Princeton University Press, Princeton
Georges J-Y, Guinet C (2000a) Early mortality and perinatal growth in the subantarctic fur seal (Arctocephalus tropicalis) on Amsterdam Island. J Zool Lond 251:277–287
Georges J-Y, Guinet C (2000b) Maternal care in the subantarctic fur seals on Amsterdam Island. Ecology 81:295–308
Georges J-Y, Guinet C (2001) Prenatal investment in the subantarctic fur seal, Arctocephalus tropicalis. Can J Zool 79:601–609
Goebel ME, Calambokidis J (1998) Neonatal growth and behavior. In: Gentry RL (ed) Behavior and ecology of the northern fur seal. Princeton University Press, Princeton, NJ, pp 233–242
Goldsworthy SD (1992) Maternal care in three species of southern fur seal (Arctocephalus spp.). PhD Thesis, Monash University, Melbourne
Goldsworthy SD, Shaughnessy PD (1994) Breeding biology and haul-out pattern of the New Zealand fur seal, Arctocephalus forsteri, at Cape Gantheaume, South Australia. Wildl Res 21:365–376
Gosling LM (1986) Selective abortion of entire litters in the coypu: adaptive control of offspring production in relation to quality and sex. Am Nat 127:772–795
Guinet C, Roux J-P, Bonnet M, Mison V (1998) Effect of body size, body mass, and body condition on reproduction of female South African fur seals (Arctocephalus pusillus) in Namibia. Can J Zool 76:1418–1424
Guinet C, Lea M-A, Goldsworthy SD (2000) Mass change in Antarctic fur seal (Arctocephalus gazella) pups in relation to maternal characteristics at the Kerguèlen Islands. Can J Zool 78:476–483
Guinet C, Dubroca L, Lea MA, Goldsworthy S, Cherel Y, Duhamel G, Bonadonna F, Donnay J-P (2001) Spatial distribution of the foraging activity of Antarctic fur seal Arctocephalus gazella females in relation to oceanographic factors: a scale-dependent approach using geographic information systems. Mar Ecol Prog Ser 219:251–264
Harcourt RG, Schulman AM, Davis LS, Trillmich F (1995) Summer foraging by lactating female New Zealand fur seals (Arctocephalus forsteri) off Otago Peninsula, New Zealand. Can J Zool 73:678–690
Harcourt RG, Bradshaw CJA, Davis LS (2001) Summer foraging behaviour of a generalist predator, the New Zealand fur seal (Arctocephalus forsteri). Wildl Res 28:599–606
Harcourt RG, Bradshaw CJA, Dickson K, Davis LS (2002) Foraging ecology of a generalist predator, the female New Zealand fur seal. Mar Ecol Prog Ser 227:11–24
Hardy IC (1997) Possible factors influencing vertebrate sex ratios: an introductory overview. Appl Anim Behav Sci 51:217–241
Hewison AJM, Andersen M, Gaillard J-M, Linnell JDC, Delorme D (1999) Contradictory findings in studies of sex ratio variation in roe deer (Capreolus capreolus). Behav Ecol Sociobiol 45:339–348
James WH (1997) A potential mechanism for sex ratio variation in mammals. J Theor Biol 189:253–255
Johnson CN, Ritchie EG (2002) Adaptive biases in offspring sex ratios established before birth in a marsupial, the common brushtail possum Trichosurus vulpecula. Behav Ecol 13:653–665
Julliard R (2000) Sex-specific dispersal in spatially varying environments leads to habitat-dependent evolutionary stable offspring sex ratios. Behav Ecol 11:421–428
Kojola I, Eloranta E (1989) Influences of maternal body weight, age, and parity on sex ratio in semidomesticated reindeer (Rangifer t. tarandus). Evolution 43:1331–1336
Krackow S (1995) Potential mechanisms for sex ratio adjustment in mammals and birds. Biol Rev 70:225–241
Krackow S (1997) Maternal investment, sex-differentiated prospects, and the sex ratio in wild house mice. Behav Ecol Sociobiol 41:435–443
Kruuk LEB, Clutton-Brock TH, Albon SD, Pemberton JM, Guinness FE (1999) Population density affects sex ratio variation in red deer. Nature 399:459–461
Lalas C, Bradshaw CJA (2001) Folklore and chimerical numbers: review of a millennium of interaction between fur seals and humans in the New Zealand region. N Z J Mar Freshwater Res 35:477–497
Lalas C, Harcourt R (1995) Pup production of the New Zealand fur seal on Otago Peninsula, New Zealand. J R Soc N Z 25:81–88
Lalas C, Murphy B (1998) Increase in the abundance of New Zealand fur seals at the Catlins, South Island, New Zealand. Roy Soc N Z 28:287–294
Lea M-A, Hindell MA, Guinet C, Goldsworthy S (2001) Variability in the diving activity of Antarctic fur seals, Arctocephalus gazella, at Iles Kerguelen. Polar Biol 25:269–279
Lebreton J-D, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Lunn NJ, Arnould JPY (1997) Maternal investment in Antarctic fur seals: evidence for equality in the sexes. Behav Ecol Sociobiol 40:351–362
Lunn NJ, Boyd IL (1991) Pupping-site fidelity of Antarctic fur seals at Bird Island, South Georgia. J Mammal 72:202–206
Manly BFJ (1997) Randomization, bootstrap and Monte Carlo methods in biology. Chapman & Hall, London
Mattlin RH (1981) Pup growth of the New Zealand fur seal Arctocephalus forsteri on the Open Bay Islands, New Zealand. J Zool Lond 193:305–314
Mattlin RH (1987) New Zealand fur seal, Arctocephalus forsteri, within the New Zealand region. In: Croxall JP, Gentry RL (eds) Status, biology, and ecology of fur seals. National Marine Fisheries Service, Cambridge, pp 49–51
Maynard-Smith J (1980) A new theory of sexual selection. Behav Ecol Sociobiol 7:247–251
Mison-Jooste V (1999) Contribution à l'étude de la biologie des populations de l'otarie à fourrure du Cap (Arctocephalus pusillus pusillus): les soins maternels diffèrent-ils en fonction du sexe du jeune? PhD Thesis, University of Lyons
Nichols JD, Sauer JR, Pollock KH, Hestbeck JB (1992) Estimating transition probabilities for stage-based population projection matrices using capture-recapture data. Ecology 73:306–312
Ono KA, Boness DJ (1991) The influence of El Niño on mother-pup behaviour, pup ontogeny, and sex ratios in the California sea lion. In: Trillmich F, Ono KA (eds) Pinnipeds and El Niño: responses to environmental stress, vol 88. Springer, Berlin Heidelberg New York, pp 185–192
Ono KA, Boness DJ (1996) Sexual dimorphism in sea lion pups: differential maternal investment, or sex-specific differences in energy allocation? Behav Ecol Sociobiol 38:31–41
Oosthuizen WH (1991) General movements of South African (Cape) fur seals Arctocephalus pusillus pusillus from analysis of recoveries of tagged animals. S Afr J Mar Sci 11:21–29
Packer C, Collines DA, Eberley LE (2000) Problems with primate sex ratios. Philos Trans R Soc Lond B 355:1627–1635
Palmer AR (2000) Quasireplication and the contract of error: lessons from sex ratios, heritabilities and fluctuating asymmetry. Annu Rev Ecol Syst 31:441–480
Rand RW (1956) The Cape fur seal Arctocephalus pusillus (Schreber). Its general characteristics and moult. Investigational Report 21. Division of Fisheries South Africa, Cape Town
Rutberg AT (1986) Lactation and fetal sex ratios in American bison. Am Nat 127:89–94
Schaik CP van, Hrdy SB (2002) Intensity of local resource competition shapes the relationship between maternal rank and sex ratios at birth in cercopithecine primates. Am Nat 138:1555–1562
Schulman AM (1996) Individual variation in maternal care and its effects on pup growth rate in the New Zealand fur seal (Arctocephalus forsteri) on the Otago Peninsula, New Zealand. MSc Thesis, University of Otago, Dunedin
Shaughnessy PD (1993) Population estimates of the Cape fur seal Arctocephalus pusillus. 2. From tagging and recapturing. In: Boonstra HGvD (ed) Investigational Report 134. Sea Fisheries Research Unit, Cape Town
Silk JB (1983) Local resource competition and facultative adjustment of sex ratios in relation to competitive abilities. Am Nat 121:56–66
Simpson MJA, Simpson AE (1982) Birth sex ratios and social rank in rhesus monkey mothers. Nature 300:440–441
Sokal RR, Rohlf FJ (1981) Biometry. The principles and practice of statistics in biological research, 2nd edn. Freeman, New York
Stirling I (1971) Studies on the behaviour of the South Australian fur seal, Arctocephalus forsteri (Lesson). II. Adult females and pups. Aust J Zool 19:267–273
Trillmich F (1986) Maternal investment and sex-allocation in the Galapagos fur seal, Arctocephalus galapagoensis. Behav Ecol Sociobiol 19:157–164
Trillmich F (1996) Parental investment in pinnipeds. Adv Stud Behav 25:533–577
Trillmich F, Kooyman GL (2001) Field metabolic rate of lactating female Galapagos fur seals (Arctocephalus galapagoenis): the influence of offspring age and environment. Comp Biochem Physiol A 129:741–749
Trites AW (1991) Fetal growth of northern fur seals: life-history strategy and sources of variation. Can J Zool 69:2608–2617
Trites AW (1992) Fetal growth and the condition of pregnant northern fur seals off western North America from 1958 to 1972. Can J Zool 70:2125–2131
Trites AW (1993) Biased estimates of fur seal pup mass: origins and implications. J Zool Lond 229:515–525
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man 1871–1971. Aldine, Chicago, pp 136–179
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
West SA, Sheldon BC (2002) Constraints in the evolution of sex ratio adjustment. Science 295:1685–1688
Williams GC (1979) The question of adaptive sex ratio in outcrossed vertebrates. Proc R Soc Lond B 205:567–580
Acknowledgements
Research was funded by the University of Otago. We also thank Allflex New Zealand (Palmerston North) for providing the plastic tags used to identify fur seal pups, Combined Rural Traders Society (Otago) for providing field equipment, and Next Stop Backpackers (Dunedin) for providing accommodation for volunteers. We particularly thank the Department of Zoology (University of Otago), New Zealand Department of Conservation, Landcare Research New Zealand, and New Zealand Sea Adventures (Kaikoura) for logistic support. We thank A. Anderson, G. Anderson, C. Bevers, K. Barton, C. Duncan, C. Littnan, N. McNally, I. Rasmussen, B. Thomas, M. Wright and the many volunteers who assisted with data collection. We thank R.J. Barker for providing statistical advice, and the editor, F. Trillmich, and two anonymous referees for greatly improving the manuscript. All animal treatment procedures were approved by the University of Otago Animal Ethics Committee (no. 83-95) and licensed under a New Zealand Department of Conservation Permit to Take Marine Mammals (30 July 1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Trillmich
Rights and permissions
About this article
Cite this article
Bradshaw, C.J.A., Harcourt, R.G. & Davis, L.S. Male-biased sex ratios in New Zealand fur seal pups relative to environmental variation. Behav Ecol Sociobiol 53, 297–307 (2003). https://doi.org/10.1007/s00265-003-0580-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0580-9