Abstract
Purpose
Carbazochrome sodium sulfonate (CSS) is a haemostatic agent. However, its hemostatic and anti-inflammatory effects in patients undergoing total hip arthroplasty (THA) via a direct anterior approach (DAA) are unknown. We investigated the efficacy and safety of CSS combined with tranexamic acid (TXA) in THA using DAA.
Methods
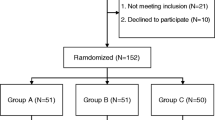
This study enrolled 100 patients who underwent primary, unilateral THA through a direct anterior approach. Patients were randomly divided into two groups: Group A used a combination of TXA and CSS, while Group B used TXA only. The primary outcome was total perioperative blood loss. The secondary outcomes were hidden blood loss, postoperative blood transfusion rate, inflammatory reactant levels, hip function, pain score, venous thromboembolism (VTE), and incidence of associated adverse reactions.
Results
The total blood loss (TBL) in group A was significantly lower than in group B. The levels of inflammatory reactants and the rate of blood transfusion were also significantly lower. However, the two groups had no significant differences in intraoperative blood loss, postoperative pain score, or joint function. There were no significant differences in VTE or postoperative complications between the groups.
Conclusion
As a haemostatic agent, CSS combined with TXA can reduce postoperative blood loss in patients undergoing THA via DAA and seems to have an anti-inflammatory effect. Moreover, it did not increase the incidence of VTE or its related complications.



Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Bourne RB, Chesworth B, Davis A, Mahomed N, Charron K (2010) Comparing patient outcomes after THA and TKA: is there a difference? Clin Orthop Relat Res 468(2):542–546. https://doi.org/10.1007/s11999-009-1046-9
Sloan M, Premkumar A, Sheth NP (2018) Projected volume of primary total joint arthroplasty in the U.S. 2014 to 2030. J Bone Joint Surg Am 100(17):1455–1460. https://doi.org/10.2106/jbjs.17.01617
Hochreiter J, Hejkrlik W, Emmanuel K, Hitzl W, Ortmaier R (2017) Blood loss and transfusion rate in short stem hip arthroplasty A comparative study. Int Orthop 41(7):1347–1353. https://doi.org/10.1007/s00264-016-3365-2
Mortazavi SMJ, Razzaghof M, Ghadimi E, Seyedtabaei SMM, Vahedian Ardakani M, Moharrami A (2022) The efficacy of bone wax in reduction of perioperative blood loss in total hip arthroplasty via direct anterior approach: a prospective randomized clinical trial. J Bone Joint Surg Am 104(20):1805–1813. https://doi.org/10.2106/jbjs.22.00376
Newman JM, Webb MR, Klika AK, Murray TG, Barsoum WK, Higuera CA (2017) Quantifying blood loss and transfusion risk after primary vs conversion total hip arthroplasty. J Arthroplasty 32(6):1902–1909. https://doi.org/10.1016/j.arth.2017.01.038
Shichman I, Shaked O, Ashkenazi I, Schwarzkopf R, Warschawski Y, Snir N (2021) Tranexamic acid in non-elective primary total hip arthroplasty. Injury. 52(6):1544–1548. https://doi.org/10.1016/j.injury.2020.10.056
Baron DM, Hochrieser H, Posch M, Metnitz B, Rhodes A, Moreno RP, Pearse RM, Metnitz P (2014) Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth 113(3):416–423. https://doi.org/10.1093/bja/aeu098
Liu X, Liu J, Sun G (2017) A comparison of combined intravenous and topical administration of tranexamic acid with intravenous tranexamic acid alone for blood loss reduction after total hip arthroplasty: a meta-analysis. Int J Surg 41:34–43. https://doi.org/10.1016/j.ijsu.2017.03.031
Paul JE, Ling E, Lalonde C, Thabane L (2007) Deliberate hypotension in orthopedic surgery reduces blood loss and transfusion requirements: a meta-analysis of randomized controlled trials. Can J Anaesth 54(10):799–810. https://doi.org/10.1007/bf03021707
Konig G, Hamlin BR, Waters JH (2013) Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty 28(9):1473–1476. https://doi.org/10.1016/j.arth.2013.06.011
Zha GC, Zhu XR, Wang L, Li HW (2022) Tranexamic acid reduces blood loss in primary total hip arthroplasty performed using the direct anterior approach: a one-center retrospective observational study. J Orthop Traumatol 23(1):12. https://doi.org/10.1186/s10195-022-00638-7
Jungwirth-Weinberger A, Do HT, Krell EC, Valle AGD, Chalmers BP, Boettner F (2021) Blood management in direct anterior versus posterior primary total hip arthroplasty using tranexamic acid: a matched cohort study. Arch Orthop Trauma Surg. https://doi.org/10.1007/s00402-021-03965-2
Good L, Peterson E, Lisander B (2003) Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth 90(5):596–599. https://doi.org/10.1093/bja/aeg111
Cha Y, Yoo JI, Kim JT, Park CH, Choy W, Ha YC, Koo KH (2020) Disadvantage during perioperative period of total hip arthroplasty using the direct anterior approach: a network meta-analysis. J Korean Med Sci 35(18):e111. https://doi.org/10.3346/jkms.2020.35.e111
Miao K, Ni S, Zhou X, Xu N, Sun R, Zhuang C, Wang Y (2015) Hidden blood loss and its influential factors after total hip arthroplasty. J Orthop Surg Res 10:36. https://doi.org/10.1186/s13018-015-0185-9
Oelsner WK, Engstrom SM, Benvenuti MA, An TJ, Jacobson RA, Polkowski GG, Schoenecker JG (2017) Characterizing the acute phase response in healthy patients following total joint arthroplasty: predictable and consistent. J Arthroplasty 32(1):309–314. https://doi.org/10.1016/j.arth.2016.06.027
Chen XX, Wang T, Li J, Kang H (2016) Relationship between inflammatory response and estimated complication rate after total hip arthroplasty. Chin Med J (Engl) 129(21):2546–2551. https://doi.org/10.4103/0366-6999.192772
Takahashi K, Sasaki T, Ueno N et al (2022) Carbazochrome sodium sulfonate is not effective for prevention of post-gastric endoscopic submucosal dissection bleeding: a retrospective study. Surg Endosc 36(10):7486–7493. https://doi.org/10.1007/s00464-022-09171-4
Luo Y, Zhao X, Yang Z, Yeersheng R, Kang P (2021) Effect of carbazochrome sodium sulfonate combined with tranexamic acid on blood loss and inflammatory response in patients undergoing total hip arthroplasty. Bone Joint Res 10(6):354–362. https://doi.org/10.1302/2046-3758.106.BJR-2020-0357.R2
Tassniyom S, Vasanawathana S, Dhiensiri T, Nisalak A, Chirawatkul A (1997) Failure of carbazochrome sodium sulfonate (AC-17) to prevent dengue vascular permeability or shock: a randomized, controlled trial. J Pediatr 131(4):525–528. https://doi.org/10.1016/s0022-3476(97)70055-6
Luo Y, Zhao X, Releken Y, Yang Z, Pei F, Kang P (2020) Hemostatic and anti-inflammatory effects of carbazochrome sodium sulfonate in patients undergoing total knee arthroplasty: a randomized controlled trial. J Arthroplasty 35(1):61–68. https://doi.org/10.1016/j.arth.2019.07.045
Sendo T, Itoh Y, Aki K, Oka M, Oishi R (2003) Carbazochrome sodium sulfonate (AC-17) reverses endothelial barrier dysfunction through inhibition of phosphatidylinositol hydrolysis in cultured porcine endothelial cells. Naunyn Schmiedebergs Arch Pharmacol 368(3):175–180. https://doi.org/10.1007/s00210-003-0785-5
Sendo T, Goromaru T, Aki K, Sakai N, Itoh Y, Oishi R (2002) Carbazochrome attenuates pulmonary dysfunction induced by a radiographic contrast medium in rats. Eur J Pharmacol 450(2):203–208. https://doi.org/10.1016/s0014-2999(02)02120-9
Onodera T, Majima T, Sawaguchi N, Kasahara Y, Ishigaki T, Minami A (2012) Risk of deep venous thrombosis in drain clamping with tranexamic acid and carbazochrome sodium sulfonate hydrate in total knee arthroplasty. J Arthroplasty 27(1):105–108. https://doi.org/10.1016/j.arth.2011.02.004
Nadler SB, Hidalgo JH, Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51(2):224–232
Fillingham YA, Ramkumar DB, Jevsevar DS et al (2018) The efficacy of tranexamic acid in total hip arthroplasty: a network meta-analysis. J Arthroplasty 33(10):3083–3089.e4. https://doi.org/10.1016/j.arth.2018.06.023
Luo Y, Releken Y, Yang D, Yue Y, Liu Z, Kang P (2022) Effects of carbazochrome sodium sulfonate combined with tranexamic acid on hemostasis and inflammation during perioperative period of total hip arthroplasty: a randomized controlled trial. Orthop Traumatol Surg Res 108(1):103092. https://doi.org/10.1016/j.otsr.2021.103092
Zhao H, Xiang M, Xia Y, Shi X, Pei FX, Kang P (2018) Efficacy of oral tranexamic acid on blood loss in primary total hip arthroplasty using a direct anterior approach: a prospective randomized controlled trial. Int Orthop 42(11):2535–2542. https://doi.org/10.1007/s00264-018-3846-6
Conlon NP, Bale EP, Herbison GP, Mccarroll M (2008) Postoperative anemia and quality of life after primary hip arthroplasty in patients over 65 years old. Anesth Analg 106(4):1056–1061. https://doi.org/10.1213/ane.0b013e318164f114
Ghadimi K, Levy JH, Welsby IJ (2016) Perioperative management of the bleeding patient. Br J Anaesth 117:iii18–iii30. https://doi.org/10.1093/bja/aew358
Donovan RL, Lostis E, Jones I, Whitehouse MR (2021) Estimation of blood volume and blood loss in primary total hip and knee replacement: an analysis of formulae for perioperative calculations and their ability to predict length of stay and blood transfusion requirements. J Orthop 24:227–232. https://doi.org/10.1016/j.jor.2021.03.004
Gibon E, Courpied JP, Hamadouche M (2013) Total joint replacement and blood loss: what is the best equation? Int Orthop 37(4):735–739. https://doi.org/10.1007/s00264-013-1801-0
Cao G, Huang Q, Huang Z, Zhang S, Luo Z, Lei Y, Zhou Z, Pei F (2019) The efficacy and safety of multiple-dose oral tranexamic acid on blood loss following total hip arthroplasty: a randomized controlled trial. Int Orthop 43(2):299–305. https://doi.org/10.1007/s00264-018-3925-8
Vles GF, Corten K, Driesen R, Van Elst C, Ghijselings SG (2021) Hidden blood loss in direct anterior total hip arthroplasty: a prospective, double blind, randomized controlled trial on topical versus intravenous tranexamic acid. Musculoskelet Surg 105(3):267–273. https://doi.org/10.1007/s12306-020-00652-0
Li B, Liu ZT, Shen P, Zhou BZ, Bai LH (2015) Comparison of therapeutic effects between drainage blood reinfusion and temporary clamping drainage after total knee arthroplasty in patients with rheumatoid arthritis. Clinics (Sao Paulo) 70(3):202–206. https://doi.org/10.6061/clinics/2015(03)09
Changjun C, Xin Z, Yue L, Chengcheng Z, Qiuru W, Qianhao L, Pengde K (2021) Tranexamic acid attenuates early post-operative systemic inflammatory response and nutritional loss and avoids reduction of fibrinogen in total hip arthroplasty within an enhanced recovery after surgery pathway. Int Orthop 45(11):2811–2818. https://doi.org/10.1007/s00264-021-05182-3
Prudovsky I, Kacer D, Zucco VV, Palmeri M, Falank C, Kramer R, Carter D, Rappold J (2022) Tranexamic acid: beyond antifibrinolysis. Transfusion 62(Suppl 1):S301–s312. https://doi.org/10.1111/trf.16976
Beverly A, Kaye AD, Ljungqvist O, Urman RD (2017) Essential elements of multimodal analgesia in enhanced recovery after surgery (ERAS) guidelines. Anesthesiol Clin 35(2):e115–e143. https://doi.org/10.1016/j.anclin.2017.01.018
Lovell TP (2008) Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplasty 23(7 Suppl):64–68. https://doi.org/10.1016/j.arth.2008.06.027
Sang W, Zhu L, Ma J, Lu H, Wang C (2016) The influence of body mass index and hip anatomy on direct anterior approach total hip replacement. Med Princ Pract 25(6):555–560. https://doi.org/10.1159/000447455
Acknowledgements
We want to express our sincere appreciation for all the patients that joined this study.
Funding
This study was funded by 1.3.5 Project of Sichuan University West China Hospital, grant no. ZYJC18040.
Author information
Authors and Affiliations
Contributions
YSW and CMJ were responsible for data collection, data analysis and manuscript writing. LY, ZCC, and LQH were responsible for data collection. KPD was responsible for the study design and correspondence. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Clinical Trials and Biomedical Ethics Committee of West China Hospital.
Consent to publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, S., Chen, M., Luo, Y. et al. Comparative study of carbazochrome sodium sulfonate and tranexamic acid in reducing blood loss and inflammatory response following direct anterior total hip arthroplasty: a prospective randomized controlled trial. International Orthopaedics (SICOT) 47, 2553–2561 (2023). https://doi.org/10.1007/s00264-023-05853-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-023-05853-3