Abstract
Purpose
Aiming to prevent cartilage damage during early osteoarthritis (OA), the therapeutic challenge is to restore and maintain the physiological and functional properties of such a tissue with minimally invasive therapeutic strategies.
Methods
Accordingly, an in vivo model of early OA in sheep was here treated through three different cell therapies (culture expanded ADSCs, SVF, and culture expanded AECs) thus to preserve the joint surface from the progression of the pathology. Three months after the treatment injections, their performance was assessed through mechanical automated mapping (Young’s modulus and cartilage thickness), gross evaluation of articular surfaces, and biochemical analysis of the synovial fluid.
Results
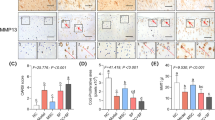
No severe degeneration was observed after three months from OA induction. Cartilage mechanical properties were crucial to identify early degeneration. All the treatments improved the macroscopic cartilage surface aspect and reduced pro-inflammatory cytokines in the synovial fluid. Among the three treatments, SVF highlighted the best performance while ADSCs the worst.
Conclusion
Despite that the evaluated experimental time is an early follow-up and, thus, longer trial is mandatory to properly assess treatments effectiveness, the proposed multidisciplinary approach allowed to obtain preliminary, but also crucial, results concerning the reduction in OA signs on cartilage properties, in osteophyte development and in all the inflammatory markers.





Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Deveza LA, Melo L, Yamato TP et al (2017) Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthr Cartil 25:1926–1941. https://doi.org/10.1016/j.joca.2017.08.009
Goldring MB, Otero M (2011) Inflammation in osteoarthritis. Curr Opin Rheumatol 23:471–478. https://doi.org/10.1097/BOR.0b013e328349c2b1
Pagani S, Borsari V, Veronesi F et al (2017) Increased chondrogenic potential of mesenchymal cells from adipose tissue versus bone marrow-derived cells in osteoarthritic in vitro models. J Cell Physiol 232:1478–1488. https://doi.org/10.1002/jcp.25651
Oakley SP, Lassere MN, Portek I et al (2004) Biomechanical, histologic and macroscopic assessment of articular cartilage in a sheep model of osteoarthritis. Osteoarthr Cartil 12:667–679
He WW, Kuang MJ, Zhao J et al (2017) Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: a meta-analysis. Int J Surg 39:95–103. https://doi.org/10.1016/j.ijsu.2017.01.087
Dall'Oca C, Cengarle M, Costanzo A et al (2017) Current concepts in treatment of early knee osteoarthritis and osteochondral lesions; the role of biological augmentations. Acta Biomed 88:5–10. https://doi.org/10.23750/abm.v88i4-S.6788
Schiavone Panni A, Vasso M, Braile A, Toro G, De Cicco A, Viggiano D, Lepore F (2019) Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: identification of a subpopulation with greater response. Int Orthop 43(1):7–13. https://doi.org/10.1007/s00264-018-4182-6
Shi WJ, Tjoumakaris FP, Lendner M, Freedman KB (2017) Biologic injections for osteoarthritis and articular cartilage damage: can we modify disease? Phys Sportsmed 45(3):203–223. https://doi.org/10.1080/00913847.2017.1357421
Ude CC, Sulaiman SB, Min-Hwei N et al (2014) Cartilage regeneration by chondrogenic induced adult stem cells in osteoarthritic sheep model. PLoS One 9(6):e98770. https://doi.org/10.1371/journal.pone.0098770
Hernigou P, Auregan JC, Dubory A, Flouzat-Lachaniette CH, Chevallier N, Rouard H (2018) (2018) Subchondral stem cell therapy versus contralateral total knee arthroplasty for osteoarthritis following secondary osteonecrosis of the knee. Int Orthop 42(11):2563–2571. https://doi.org/10.1007/s00264-018-3916-9
Roato I, Belisario DC, Compagno M, Lena A, Bistolfi A, Maccari L, Mussano F, Genova T, Godio L, Perale G, Formica M, Cambieri I, Castagnoli C, Robba T, Felli L, Ferracini R (2019) Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: clinical and histological observations. Int Orthop 43(1):15–23. https://doi.org/10.1007/s00264-018-4192-4
Hong Z, Chen J, Zhang S, Zhao C, Bi M, Chen X, Bi Q (2019) Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop 43(5):1123–1134. https://doi.org/10.1007/s00264-018-4099-0
Pas HI, Winters M, Haisma HJ et al (2017) Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med 51(15):1125–1133. https://doi.org/10.1136/bjsports-2016-096793
Damia E, Chicharro D, Lopez S et al (2018) Adipose-derived mesenchymal stem cells: are they a good therapeutic strategy for osteoarthritis? Int J Mol Sci 19. https://doi.org/10.3390/ijms19071926
Bansal H, Comella K, Leon J et al (2017) Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med 15:141. https://doi.org/10.1186/s12967-017-1242-4
Hurley ET, Yasui Y, Gianakos AL et al (2018) Limited evidence for adipose-derived stem cell therapy on the treatment of osteoarthritis. Knee Surg Sports Traumatol Arthrosc 26:3499–3507. https://doi.org/10.1007/s00167-018-4955-x
Marchiori G, Berni M, Boi M, Filardo G (2019) Cartilage mechanical tests: evolution of current standards for cartilage repair and tissue engineering. A literature review. Clin Biomech 68:58–72. https://doi.org/10.1016/j.clinbiomech.2019.05.019
Boi M, Marchiori G, Berni M et al (2019) Nanoindentation: an advanced procedure to investigate osteochondral engineered tissues. J Mech Behav Biomed Mater 96:79–87. https://doi.org/10.1016/j.jmbbm.2019.04.042
Delling U, Brehm W, Ludewig E et al (2015) Longitudinal evaluation of effects of intra-articular mesenchymal stromal cell administration for the treatment of osteoarthritis in an ovine model. Cell Transplant 24:2391–2407. https://doi.org/10.3727/096368915X686193
Little CB, Smith MM, Cake MA et al (2010) The OARSI histopathology initiative e recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthr Cartil 18:S80–S92. https://doi.org/10.1016/j.joca.2010.04.016
Sim S, Chevrier A, Garon M et al (2017) Electromechanical probe and automated indentation maps are sensitive techniques in assessing early degenerated human articular cartilage. J Orthop Res 35:858–867. https://doi.org/10.1002/jor.23330
Cook JL, Hung CT, Kuroki K et al (2014) Animal models of cartilage repair. Bone Joint Res 3:89–94. https://doi.org/10.1302/2046-3758.34.2000238
Feng C, Luo X, He N et al (2018) Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a sheep model. Tissue Eng Part A 24:219–233. https://doi.org/10.1089/ten.TEA.2017.0039
Cake MA, Read RA, Corfield G et al (2013) Comparison of gait and pathology outcomes of three meniscal procedures for induction of knee osteoarthritis in sheep. Osteoarthr Cartil 21:226–236. https://doi.org/10.1016/j.joca.2012.10.001
Nguyen LT, Sharma AR, Chakraborty C et al (2017) Review of prospects of biological fluid biomarkers in osteoarthritis. Int J Mol Sci 18:601. https://doi.org/10.3390/ijms18030601
Temple-Wong MM, Bae WC, Chen MQ et al (2009) Biomechanical, structural, and biochemical indices of degenerative and osteoarthritic deterioration of adult human articular cartilage of the femoral condyle. Osteoarthr Cartil 17:1469–1476. https://doi.org/10.1016/j.joca.2009.04.017
Boettcher K, Kienle S, Nachtsheim J et al (2016) The structure and mechanical properties of articular cartilage are highly resilient towards transient dehydration. Acta Biomater 29:180–187. https://doi.org/10.1016/j.actbio.2015.09.034
Setton LA, Elliott DM, Mow VC (1999) Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthr Cartil 7:2–14
Middendorf JM, Griffin DJ, Shortkroff S et al (2017) Mechanical properties and structure-function relationships of human chondrocyte-seeded cartilage constructs after in vitro culture. J Orthop Res 35:2298–2306. https://doi.org/10.1002/jor.23535
Korhonen RK, Laasanen MS, Toyras J et al (2002) Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech 35:903–909
Armstrong SJ, Readn RA, Price R (1995) Topographical variation within the articular cartilage and subchondral bone of the normal ovine knee joint: a histological approach. Osteoarthr Cartil 3:25–33
Lammentausta E, Kiviranta P, Töyräs J et al (2007) Quantitative MRI of parallel changes of articular cartilage and underlying trabecular bone in degeneration. Osteoarthr Cartil 15:1149–1157
Kleemann RU, Schell H, Thompson M et al (2007) Mechanical behavior of articular cartilage after osteochondral autograft transfer in an ovine model. Am J Sports Med 35:555–563
Pearle AD, Warren RF, Rodeo SA (2005) Basic science of articular cartilage and osteoarthritis. Clin Sports Med 24(1):1–12
Panula HE, Hyttinen MM, Arokoski JPA et al (1998) Articular cartilage superficial zone collagen birefringence reduced and cartilage thickness increased before surface fibrillation in experimental osteoarthritis. Ann Rheum Dis 57:237–245
Funding
This work was partially supported by the Ministry of Health-Ricerca Corrente to the Rizzoli Orthopedic Institute and by PRIN: PROGETTI DI RICERCA DI RILEVANTE INTERESSE NAZIONALE—Bando 2015 (Amniotic epithelial stem cells (AECs) vs adipose-derived mesenchymal stem cells (ADSCs): translational potential as biological injective treatment for osteoarthritis).
Author information
Authors and Affiliations
Contributions
Conceptualization: Francesca Veronesi, Matteo Berni, Gregorio Marchiori, Milena Fini, Elizaveta Kon;
Methodology: Giorgio Cassiolas, Aurelio Muttini, Barbara Barboni, Lucia Martini;
Formal analysis and investigation: Francesca Veronesi, Matteo Berni, Gregorio Marchiori;
Writing - original draft preparation: Francesca Veronesi, Matteo Berni, Gregorio Marchiori;
Writing - review and editing: Milena Fini, Elizaveta Kon, Maurilio Marcacci;
Supervision: Milena Fini, Elizaveta Kon, Maurilio Marcacci.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was conducted in accordance with the European and Italian Law on animal experiments. The research protocol was approved (N.62/2018-PR date 29/01/2018) by the Ethical Committee of Rizzoli Orthopedic Institute and by the responsible public authorities in agreement with EU regulations (EU Directive 2010/63/EU for animal experiments).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 128 kb).
Rights and permissions
About this article
Cite this article
Veronesi, F., Berni, M., Marchiori, G. et al. Evaluation of cartilage biomechanics and knee joint microenvironment after different cell-based treatments in a sheep model of early osteoarthritis. International Orthopaedics (SICOT) 45, 427–435 (2021). https://doi.org/10.1007/s00264-020-04701-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-020-04701-y