Abstract
Purpose
We aimed to assess osteoclastogenic potential of peripheral blood mononuclear cells (PBMC) and synovial fluid-derived mononuclear cells (SFMC) in different forms of arthritis and to correlate it with inflammatory mediators within intra-articular and circulatory compartments.
Methods
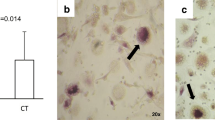
Paired PBMC and SFMC samples of patients with rheumatoid arthritis (RA; n = 10) and psoriatic arthritis (PsA; n = 10), and PBMC of healthy controls were cultured to assess osteoclastogenic potential by the number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts (OCs) and expression of OC-related genes (receptor activator of nuclear factor-κΒ (RANK), cFMS, and TRAP). Osteoclastogenesis was correlated with the arthritis-related inflammatory indicators in serum and synovial fluid (SF).
Results
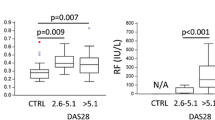
Number of OCs differentiated from PBMC was significantly higher in RA and PsA compared with control, with RA having more OCs compared with PsA. There was no difference in SFMC OC number between arthritic patients, but RANK expression in OCs differentiated from SFMC was higher in PsA compared with RA. SF of PsA patients more potently induced OC differentiation from control CD3-CD19-CD56-CD11b+CD115+ PBMC compared with RA, paralleled with higher RANK-ligand expression in PsA SFMC. Positive correlations of OC number with erythrocyte sedimentation rate, serum level of CCL2, and PBMC gene expression of interleukin-18 and Fas-ligand were observed.
Conclusion
Osteoclastogenic potential is systemically enhanced in patients with RA, paralleled by disordered systemic and local expression of proinflammatory mediators, whereas PsA involves specific deregulation in RANKL/RANK axis. Our study reveals arthritis-specific mediators associated with the form of arthritis, indicating clinical relevance for diagnosis and treatment.





Similar content being viewed by others
References
Lari R, Kitchener PD, Hamilton JA (2009) The proliferative human monocyte subpopulation contains osteoclast precursors. Arthritis Res Ther 11:R23
Roux C (2011) Osteoporosis in inflammatory joint diseases. Osteoporos Int 22:421–433
Grčević D, Lukić IK, Kovačić N, Ivčević S, Katavić V, Marušić A (2006) Activated T lymphocytes suppress osteoclastogenesis by diverting early monocyte/macrophage progenitor lineage commitment towards dendritic cell differentiation through down-regulation of receptor activator of nuclear factor-kappaB and c-Fos. Clin Exp Immunol 146:146–158
Colucci S, Brunetti G, Cantatore FP et al (2007) Lymphocytes and synovial fluid fibroblasts support osteoclastogenesis through RANKL, TNFalpha, and IL-7 in an in vitro model derived from human psoriatic arthritis. J Pathol 212:47–55
Dalbeth N, Pool B, Smith T et al (2010) Circulating mediators of bone remodeling in psoriatic arthritis: implications for disordered osteoclastogenesis and bone erosion. Arthritis Res Ther 12:R164
Lorenzo J, Horowitz M, Choi Y (2008) Osteoimmunology: interactions of the bone and immune system. Endocr Rev 29:403–440
Grčević D, Jajić Z, Kovačić N, Lukić IK, Velagić V, Grubišić F, Ivčević S, Marušić A (2010) Peripheral blood expression profiles of bone morphogenetic proteins, tumor necrosis factor-superfamily molecules, and transcription factor Runx2 could be used as markers of the form of arthritis, disease activity, and therapeutic responsiveness. J Rheumatol 37:246–256
Schett G, Saag KG, Bijlsma JW (2010) From bone biology to clinical outcome: state of the art and future perspectives. Ann Rheum Dis 69:1415–1419
Komano Y, Nanki T, Hayashida K, Taniguchi K, Miyasaka N (2006) Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res Ther 8:R152
Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM (2003) Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest 111:821–831
Bugatti S, Manzo A, Bombardieri M, Vitolo B, Humby F, Kelly S, Montecucco C, Pitzalis C (2011) Synovial tissue heterogeneity and peripheral blood biomarkers. Curr Rheumatol Rep 13:440–448
Finzel S, Englbrecht M, Engelke K, Stach C, Schett G (2011) A comparative study of periarticular bone lesions in rheumatoid arthritis and psoriatic arthritis. Ann Rheum Dis 70:122–127
Miyamoto K, Ninomiya K, Sonoda KH et al (2009) MCP-1 expressed by osteoclasts stimulates osteoclastogenesis in an autocrine/paracrine manner. Biochem Biophys Res Commun 383:373–377
Kim MS, Magno CL, Day CJ, Morrison NA (2006) Induction of chemokines and chemokine receptors CCR2b and CCR4 in authentic human osteoclasts differentiated with RANKL and osteoclast like cells differentiated by MCP-1 and RANTES. J Cell Biochem 97:512–518
Kim MS, Day CJ, Morrison NA (2005) MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem 280:16163–16169
Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y (2001) Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem 276:563–568
Adamopoulos IE, Chao CC, Geissler R, Laface D, Blumenschein W, Iwakura Y, McClanahan T, Bowman EP (2010) Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther 12:R29
Toussirot E (2012) The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets 11:159–168
Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G (1991) Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J 10:4025–4031
Morita Y, Kitaura H, Yoshimatsu M, Fujimura Y, Kohara H, Eguchi T, Yoshida N (2010) IL-18 inhibits TNF-alpha-induced osteoclastogenesis possibly via a T cell-independent mechanism in synergy with IL-12 in vivo. Calcif Tissue Int 86:242–248
Zhang W, Cong XL, Qin YH, He ZW, He DY, Dai SM (2013) IL-18 upregulates the production of key regulators of osteoclastogenesis from fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation 36:103–109
Dai SM, Nishioka K, Yudoh K (2004) Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 beta and tumour necrosis factor alpha. Ann Rheum Dis 63:1379–1386
Park H, Jung YK, Park OJ, Lee YJ, Choi JY, Choi Y (2005) Interaction of Fas ligand and Fas expressed on osteoclast precursors increases osteoclastogenesis. J Immunol 175:7193–7201
Peng SL (2006) Fas (CD95)-related apoptosis and rheumatoid arthritis. Rheumatology (Oxford) 45:26–30
Dickerson TJ, Suzuki E, Stanecki C, Shin HS, Qui H, Adamopoulos IE (2012) Rheumatoid and pyrophosphate arthritis synovial fibroblasts induce osteoclastogenesis independently of RANKL, TNF and IL-6. J Autoimmun 39:36–76
Acknowledgments
This work was supported by grants from the Croatian Ministry of Science, Education and Sports (108-1080229-0140, 108-1080229-0142, and 108-1080229-0341).
We thank Prof. Vedran Katavic for critically revising the manuscript and Mrs. Katerina Zrinski-Petrovic for her technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 17.3 kb)
Supplementary Table 2
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Ikić, M., Jajić, Z., Lazić, E. et al. Association of systemic and intra-articular osteoclastogenic potential, pro-inflammatory mediators and disease activity with the form of inflammatory arthritis. International Orthopaedics (SICOT) 38, 183–192 (2014). https://doi.org/10.1007/s00264-013-2121-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-013-2121-0