Abstract
Purpose
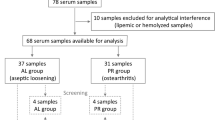
The most common long-term complication of joint arthroplasty is aseptic loosening. The proinflammatory cytokines secreted by macrophages are involved in aseptic loosening. Recently, a novel proinflammatory cytokine IL-17C was reported to participate in inflammatory diseases by synergising with proinflammatory cytokines. However, the relationship between IL-17C and the aseptic loosening is unclear.
Methods
The tissues around aseptic loosened implants were collected during revision surgery and handled by formalin fixation and embedded in paraffin. The presence of IL-17C in the tissues around the aseptic loosened implants was investigated in 12 aseptic loosening patients using immunofluorescence.
Results
The presence of IL-17C protein in the tissues around aseptic loosened implants was detected by immunofluorescence. There are no statistical differences between optical density of IL-17C in aseptic loosening samples and in rheumatoid arthritis samples (positive control).
Conclusions
These results suggest the presence of IL-17C in aseptic loosening. Interleukin-17C was related to the inflammation of aseptic loosening, possibly by contributing to the inflammation and osteolysis in the tissues surrounding aseptic loosened implants.






Similar content being viewed by others
References
Howard JL, Kremers HM, Loechler YA et al (2011) Comparative survival of uncemented acetabular components following primary total hip arthroplasty. J Bone Joint Surg Am 93(17):1597–1604. doi:10.2106/JBJS.J.00195
Della VC, Mesko NW, Quigley L et al (2009) Primary total hip arthroplasty with a porous-coated acetabular component. A concise follow-up, at a minimum of twenty years, of previous reports. J Bone Joint Surg Am 91(5):1130–1135. doi:10.2106/JBJS.H.00168
McLaughlin JR, Lee KR (2008) Total hip arthroplasty with an uncemented tapered femoral component. J Bone Joint Surg Am 90(6):1290–1296. doi:10.2106/JBJS.G.00771
Willert HG, Bertram H, Buchhorn GH (1990) Osteolysis in alloarthroplasty of the hip. The role of ultra-high molecular weight polyethylene wear particles. Clin Orthop Relat Res (258):95–107
von Knoch M, Jewison DE, Sibonga JD et al (2004) The effectiveness of polyethylene versus titanium particles in inducing osteolysis in vivo. J Orthop Res 22(2):237–243. doi:10.1016/j.orthres.2003.08.013
Tatro JM, Taki N, Islam AS et al (2007) The balance between endotoxin accumulation and clearance during particle-induced osteolysis in murine calvaria. J Orthop Res 25(3):361–369. doi:10.1002/jor.20289
Bi Y, Seabold JM, Kaar SG, Bi Y, Seabold JM, Kaar SG et al (2001) Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res 16(11):2082–2091. doi:10.1359/jbmr.2001.16.11.2082
Cho DR, Shanbhag AS, Hong CY et al (2002) The role of adsorbed endotoxin in particle-induced stimulation of cytokine release. J Orthop Res 20(4):704–713. doi:10.1016/S0736-0266(01)00179-6
Schwab LP, Xing Z, Hasty KA et al (2006) Titanium particles and surface-bound LPS activate different pathways in IC-21 macrophages. J Biomed Mater Res B Appl Biomater 79(1):66–73. doi:10.1002/jbm.b.30512
Ren W, Li XH, Chen BD et al (2004) Erythromycin inhibits wear debris-induced osteoclastogenesis by modulation of murine macrophage NF-kappaB activity. J Orthop Res 22(1):21–29. doi:10.1016/S0736-0266(03)00130-X
Gallo J, Kaminek P, Ticha V et al (2002) Particle disease. A comprehensive theory of periprosthetic osteolysis: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 146(2):21–28
Schwarz EM, Lu AP, Goater JJ et al (2000) Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res 18(3):472–480. doi:10.1002/jor.1100180321
Tunney MM, Patrick S, Gorman SP et al (1998) Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg Br 80(4):568–572
Tunney MM, Patrick S, Curran MD et al (1999) Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol 37(10):3281–3290
Clarke MT, Roberts CP, Lee PT et al (2004) Polymerase chain reaction can detect bacterial DNA in aseptically loose total hip arthroplasties. Clin Orthop Relat Res (427):132–137
Savarino L, Baldini N, Tarabusi C et al (2004) Diagnosis of infection after total hip replacement. J Biomed Mater Res B Appl Biomater 70(1):139–145. doi:10.1002/jbm.b.30030
Nalepka JL, Lee MJ, Kraay MJ et al (2006) Lipopolysaccharide found in aseptic loosening of patients with inflammatory arthritis. Clin Orthop Relat Res 451:229–235. doi:10.1097/01.blo.0000224050.94248.38
Maloney WJ, James RE, Smith RL (1996) Human macrophage response to retrieved titanium alloy particles in vitro. Clin Orthop Relat Res (322):268–278
Hallab NJ, Jacobs JJ (2009) Biologic effects of implant debris. Bull NYU Hosp Jt Dis 67(2):182–188
Ingham E, Green TR, Stone MH et al (2000) Production of TNF-alpha and bone resorbing activity by macrophages in response to different types of bone cement particles. Biomaterials 21(10):1005–1013
Nakashima Y, Sun DH, Trindade MC et al (1999) Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J Bone Joint Surg Am 81(5):603–615
Li H, Chen J, Huang A et al (2000) Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci USA 97(2):773–778
Johansen C, Riis JL, Gedebjerg A et al (2011) Tumor necrosis factor alpha-mediated induction of interleukin 17C in human keratinocytes is controlled by nuclear factor kappa B. J Biol Chem 286(29):25487–25494
Johansen C, Vinter H, Soegaard-Madsen L et al (2010) Preferential inhibition of the mRNA expression of p38 mitogen-activated protein kinase regulated cytokines in psoriatic skin by anti-TNFalpha therapy. Br J Dermatol 163(6):1194–1204. doi:10.1111/j.1365-2133.2010.10036.x
Zhang F, Wang CL, Koyama Y et al (2010) Compressive force stimulates the gene expression of IL-17s and their receptors in MC3T3-E1 cells. Connect Tissue Res 51(5):359–369
Yamaguchi Y, Fujio K, Shoda H et al (2007) IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J Immunol 179(10):7128–7136
Wu Q, Martin RJ, Rino JG et al (2007) IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect 9(1):78–86
Hwang SY, Kim HY (2005) Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Mol Cells 19(2):180–184
Johansen C, Usher PA, Kjellerup RB et al (2009) Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol 160(2):319–324. doi:10.1111/j.1365-2133.2008.08902.x
Robertson D, Savage K, Reis-Filho JS et al (2008) Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol 9:13. doi:10.1186/1471-2121-9-13
Long DN, Buggs C (2008) Microwave oven-based technique for immunofluorescent staining of paraffin-embedded tissues. J Mol Histol 39(1):1–4. doi:10.1007/s10735-007-9093-6
Purdue PE, Koulouvaris P, Potter HG et al (2007) The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res 454:251–261. doi:10.1097/01.blo.0000238813.95035.1b
Abu-Amer Y, Darwech I, Clohisy JC (2007) Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res Ther 9(Suppl 1):S6
Ramirez-Carrozzi V, Sambandam A, Luis E et al (2011) IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 12(12):1159–1166. doi:10.1038/ni.2156
Acknowledgments
This work was funded by the National Natural Science Foundation of China (81171710) (http://www.nsfc.gov.cn/Portal0/default166.htm), the Industrialisation of New and High Technology Projects–Industrial Research of Guangdong Province, China (2011B010500012), and the International Science and Technology Cooperation of Science and Technology of Guangdong Province, China (2010B050300009) (http://www.gdstc.gov.cn/eng/mission.html). The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We sincerely thank Mr. Chaohong Li (Zhongshan Medical school of Sun Yat-sen University, Guangzhou, China) for his technical guidance and device support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, C., Zhang, Y., Yu, S. et al. Presence of interleukin-17C in the tissue around aseptic loosened implants. International Orthopaedics (SICOT) 37, 953–959 (2013). https://doi.org/10.1007/s00264-013-1812-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-013-1812-x