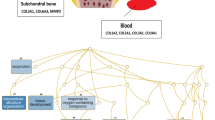
Abstract
Rapid advancements in the field of genomics, enabled by the achievements of the Human Genome Project and the complete decoding of the human genome, have opened an unimaginable set of opportunities for scientists to further unveil delicate mechanisms underlying the functional homeostasis of biological systems. The trend of applying whole-genome analysis techniques has also contributed to a better understanding of physiological and pathological processes involved in homeostasis of bone and cartilage tissues. Gene expression profiling studies have yielded novel insights into the complex interplay of osteoblast and osteoclast regulation, as well as paracrine and endocrine control of bone and cartilage remodelling. Mechanisms of new bone formation responsible for fracture healing and distraction osteogenesis, as well as healing of joint cartilage defects, have also been extensively studied. Microarray experiments have been especially useful in studying pathological processes involved in diseases such as osteoporosis or bone tumours. Existing results show that microarrays hold great promise in areas such as identification of targets for novel therapies or development of new biomarkers and classifiers in skeletal diseases.
Résumé
Les progrès rapides réalisés dans le cadre de la génétique nous ont permis d’achever le projet de génome humain et de compléter son décodage, ceci nous a permis de mieux comprendre également la physiologie et la pathologie de l’homéostasie des tissus osseux cartilagineux, l’expression des gênes interférant sur la régulation des ostéoclastes ou du remodelage osseux par l’intermédiaire d’un contrôle paracrine et endocrine. De même, en ce qui concerne les mécanismes responsables de la consolidation des fractures, de l’ostéogénèse en distraction, de la cicatrisation des lésions cartilagineuses. Ces classifications et ces expérimentations sont également utiles pour comprendre les processus pathologiques tel que l’ostéoporose ou les tumeurs osseuses. Ceci permettra de mettre en route de nouvelles thérapeutiques ou de développer de nouveaux marqueurs afin de pouvoir classer les lésions osseuses.

Similar content being viewed by others
Notes
The genome is the complete set of sequences in the genetic material of an organism. It includes the sequences of each chromosome plus any DNA in organelles.
The proteome is the complete set of proteins that is expressed by the entire genome. Because some genes code for multiple proteins, the size of the proteome is greater than the number of genes.
References
Beck GR Jr, Zerler B, Moran E (2001) Gene array analysis of osteoblast differentiation. Cell Growth Differ 12:61–83
Cappellen D, Luong-Nguyen NH, Bongiovanni S, Grenet O, Wanke C, Susa M (2002) Transcriptional program of mouse osteoclast differentiation governed by the macrophage colony-stimulating factor and the ligand for the receptor activator of NFkappa B. J Biol Chem 277:21971–21982
Carvalho RS, Einhorn TA, Lehmann W, Edgar C, Al-Yamani A, Apazidis A, Pacicca D, Clemens TL, Gerstenfeld LC (2004) The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone 34:849–861
Chandar N, Logan D, Szajkovics A, Harmston W (2004) Gene expression changes accompanying p53 activity during estrogen treatment of osteoblasts. Life Sci 75:2045–2055
Dalla-Torre CA, Yoshimoto M, Lee CH, Joshua AM, de Toledo SR, Petrilli AS, Andrade JA, Chilton-MacNeill S, Zielenska M, Squire JA (2006) Effects of THBS3, SPARC and SPP1 expression on biological behavior and survival in patients with osteosarcoma. BMC Cancer 6:237
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13:156–163
Glass DA, Karsently G (2007) In vivo analysis of Wnt signaling in bone. Endocrinology. DOI 10.1210/en.2006-1372
Goldring SR, Goldring MB (2007) Eating bone or adding it: the Wnt pathway decides. Nat Med 13:133–134
Hameetman L, Rozeman LB, Lombaerts M, Oosting J, Taminiau AH, Cleton-Jansen AM, Bovee JV, Hogendoorn PC (2006) Peripheral chondrosarcoma progression is accompanied by decreased Indian Hedgehog signalling. J Pathol 209:501–511
Heidenblad M, Hallor KH, Staaf J, Jonsson G, Borg A, Hoglund M, Mertens F, Mandahl N (2006) Genomic profiling of bone and soft tissue tumors with supernumerary ring chromosomes using tiling resolution bacterial artificial chromosome microarrays. Oncogene 25:7106–7116
Ishida N, Hayashi K, Hoshijima M, Ogawa T, Koga S, Miyatake Y, Kumegawa M, Kimura T, Takeya T (2002) Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem 277:41147–41156
Kalajzic I, Staal A, Yang WP, Wu Y, Johnson SE, Feyen JH, Krueger W, Maye P, Yu F, Zhao Y, Kuo L, Gupta RR, Achenie LE, Wang HW, Shin DG, Rowe DW (2005) Expression profile of osteoblast lineage at defined stages of differentiation. J Biol Chem 280:24618–24626
Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES (2004) Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science 303:229–232
Koga T, Matsui Y, Asagiri M, Kodama T, de Crombrugghe B, Nakashima K, Takayanagi H (2005) NFAT and Osterix cooperatively regulate bone formation. Nat Med 11:880–885
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 30:755–764
Lind M, Bunger C (2005) Orthopaedic applications of gene therapy. Int Orthop 29:205–209
Lindberg MK, Moverare S, Eriksson AL, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C (2002) Identification of estrogen-regulated genes of potential importance for the regulation of trabecular bone mineral density. J Bone Miner Res 17:2183–2195
Liu YZ, Dvornyk V, Lu Y, Shen H, Lappe JM, Recker RR, Deng HW (2005) A novel pathophysiological mechanism for osteoporosis suggested by an in vivo gene expression study of circulating monocytes. J Biol Chem 280:29011–29016
Mintz MB, Sowers R, Brown KM, Hilmer SC, Mazza B, Huvos AG, Meyers PA, Lafleur B, McDonough WS, Henry MM, Ramsey KE, Antonescu CR, Chen W, Healey JH, Daluski A, Berens ME, Macdonald TJ, Gorlick R, Stephan DA (2005) An expression signature classifies chemotherapy-resistant pediatric osteosarcoma. Cancer Res 65:1748–1754
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29
Ohali A, Avigad S, Zaizov R, Ophir R, Horn-Saban S, Cohen IJ, Meller I, Kollender Y, Issakov J, Yaniv I (2004) Prediction of high risk Ewing’s sarcoma by gene expression profiling. Oncogene 23:8997–9006
Orlic I, Borovecki F, Simic P, Vukicevic S (2007) Gene expression profiling in bone tissue of osteoporotic mice. Arh Hig Rada Toksikol 58:3–11
Pacicca DM, Patel N, Lee C, Salisbury K, Lehmann W, Carvalho R, Gerstenfeld LC, Einhorn TA (2003) Expression of angiogenic factors during distraction osteogenesis. Bone 33:889–898
Pecina M, Jelic M, Ivkovic A, Hudetz D (2006) Gene therapy applications in orthopaedics. Int Orthop 30:215–216
Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S (2002) Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop 26:131–136
Qi H, Aguiar DJ, Williams SM, La Pean A, Pan W, Verfaillie CM (2003) Identification of genes responsible for osteoblast differentiation from human mesodermal progenitor cells. Proc Natl Acad Sci U S A 100:3305–3310
Reppe S, Stilgren L, Olstad OK, Brixen K, Nissen-Meyer LS, Gautvik KM, Abrahamsen B (2006) Gene expression profiles give insight into the molecular pathology of bone in primary hyperparathyroidism. Bone 39:189–198
Rozeman LB, Szuhai K, Schrage YM, Rosenberg C, Tanke HJ, Taminiau AH, Cleton-Jansen AM, Bovee JV, Hogendoorn PC (2006) Array-comparative genomic hybridization of central chondrosarcoma: identification of ribosomal protein S6 and cyclin-dependent kinase 4 as candidate target genes for genomic aberrations. Cancer 107:380–388
Rundle CH, Wang H, Yu H, Chadwick RB, Davis EI, Wergedal JE, Lau KH, Mohan S, Ryaby JT, Baylink DJ (2006) Microarray analysis of gene expression during the inflammation and endochondral bone formation stages of rat femur fracture repair. Bone 38:521–529
Siligan C, Ban J, Bachmaier R, Spahn L, Kreppel M, Schaefer KL, Poremba C, Aryee DN, Kovar H (2005) EWS-FLI1 target genes recovered from Ewing’s sarcoma chromatin. Oncogene 24:2512–2524
Stains JP, Civitelli R (2003) Genomic approaches to identifying transcriptional regulators of osteoblast differentiation. Genome Biol 4:222.1–222.4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borovecki, F., Pecina-Slaus, N. & Vukicevic, S. Biological mechanisms of bone and cartilage remodelling—genomic perspective. International Orthopaedics (SICO 31, 799–805 (2007). https://doi.org/10.1007/s00264-007-0408-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-007-0408-8