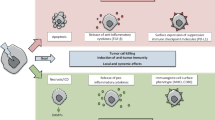
Abstract
Radiation is commonly used as a treatment intended to cure or palliate cancer patients. Despite remarkable advances in the precision of radiotherapy delivery, even the most advanced forms inevitably expose some healthy tissues surrounding the target site to radiation. On rare occasions, this results in the development of radiation-associated secondary malignancies (RASM). RASM are typically high-grade and carry a poorer prognosis than their non-radiated counterparts. RASM are characterized by a high mutation burden, increased T cell infiltration, and a microenvironment that bears unique inflammatory signatures of prior radiation, including increased expression of various cytokines (e.g., TGF-β, TNF-α, IL4, and IL10). Interestingly, these cytokines have been shown to up-regulate the expression of PD-1 and/or PD-L1—an immune checkpoint receptor/ligand pair that is commonly targeted by immune checkpoint blocking immunotherapies. Here, we review the current understanding of the tumor-immune interactions in RASM, highlight the distinct clinical and molecular characteristics of RASM that may render them immunologically “hot,” and propose a rationale for the formal testing of immune checkpoint blockade as a treatment approach for patients with RASM.

Similar content being viewed by others
References
Ravanat JL, Breton J, Douki T, Gasparutto D, Grand A, Rachidi W et al (2014) Radiation-mediated formation of complex damage to DNA: a chemical aspect overview. Br J Radiol 87(1035):20130715
Delaney G, Jacob S, Featherstone C, Barton M (2005) The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104(6):1129–1137
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V et al (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366(9503):2087–2106
Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M et al (2010) Subsequent neoplasms in 5-year survivors of childhood cancer: the childhood cancer survivor study. J Natl Cancer Inst 102(14):1083–1095
Brenner DJ, Curtis RE, Hall EJ, Ron E (2000) Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer 88(2):398–406
Morton LM, Onel K, Curtis RE, Hungate EA, Armstrong GT (2014) The rising incidence of second cancers: patterns of occurrence and identification of risk factors for children and adults. Am Soc Clin Oncol Edu Book. https://doi.org/10.14694/EdBook_AM.2014.34.e57
Berrington de Gonzalez A, Curtis RE, Kry SF, Gilbert E, Lamart S, Berg CD et al (2011) Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol 12(4):353–360
Cahan WG, Woodard HQ et al (1948) Sarcoma arising in irradiated bone; report of 11 cases. Cancer 1(1):3–29
Yeang MS, Tay K, Ong WS, Thiagarajan A, Tan DS, Ha TC et al (2013) Outcomes and prognostic factors of post-irradiation and de novo sarcomas of the head and neck: a histologically matched case-control study. Ann Surg Oncol 20(9):3066–3075
Gladdy RA, Qin LX, Moraco N, Edgar MA, Antonescu CR, Alektiar KM et al (2010) Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol 28(12):2064–2069
Bjerkehagen B, Smeland S, Walberg L, Skjeldal S, Hall KS, Nesland JM et al (2008) Radiation-induced sarcoma: 25-year experience from the Norwegian Radium Hospital. Acta Oncol 47(8):1475–1482
Tay GC, Iyer NG, Ong WS, Tai D, Ang MK, Ha TC et al (2016) Outcomes and prognostic factors of radiation-induced and de novo head and neck squamous cell carcinomas. Otolaryngol Head Neck Surg 154(5):880–887
Lee JS, DuBois SG, Coccia PF, Bleyer A, Olin RL, Goldsby RE (2016) Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer 122(1):116–123
MacArthur AC, Spinelli JJ, Rogers PC, Goddard KJ, Phillips N, McBride ML (2007) Risk of a second malignant neoplasm among 5-year survivors of cancer in childhood and adolescence in British Columbia. Canada Pediatr Blood Cancer 48(4):453–459
Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M et al (2009) Second neoplasms in survivors of childhood cancer: findings from the childhood cancer survivor study cohort. J Clin Oncol 27(14):2356–2362
Armstrong GT, Liu W, Leisenring W, Yasui Y, Hammond S, Bhatia S et al (2011) Occurrence of multiple subsequent neoplasms in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 29(22):3056–3064
Hall EJ (2006) Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys 65(1):1–7
Boice JD Jr, Harvey EB, Blettner M, Stovall M, Flannery JT (1992) Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med 326(12):781–785
Chaturvedi AK, Engels EA, Gilbert ES, Chen BE, Storm H, Lynch CF et al (2007) Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst 99(21):1634–1643
Berrington de Gonzalez A, Wong J, Kleinerman R, Kim C, Morton L, Bekelman JE (2015) Risk of second cancers according to radiation therapy technique and modality in prostate cancer survivors. Int J Radiat Oncol Biol Phys 91(2):295–302
Hooning MJ, Aleman BM, Hauptmann M, Baaijens MH, Klijn JG, Noyon R et al (2008) Roles of radiotherapy and chemotherapy in the development of contralateral breast cancer. J Clin Oncol 26(34):5561–5568
De Bruin ML, Sparidans J, vanʹt Veer MB, Noordijk EM, Louwman MW, Zijlstra JM et al (2009) Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 27(26):4239–4246
Xiang M, Chang DT, Pollom EL (2020) Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer 126(15):3560–3568
Sethi RV, Shih HA, Yeap BY, Mouw KW, Petersen R, Kim DY et al (2014) Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer 120(1):126–133
Mullenders L, Atkinson M, Paretzke H, Sabatier L, Bouffler S (2009) Assessing cancer risks of low-dose radiation. Nat Rev Cancer 9(8):596–604
Schneider U, Lomax A, Timmermann B (2008) Second cancers in children treated with modern radiotherapy techniques. Radiother Oncol 89(2):135–140
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867
Ikushima H, Miyazono K (2010) TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 10(6):415–424
Zhang Q, Liu J, Ao N, Yu H, Peng Y, Ou L et al (2020) Secondary cancer risk after radiation therapy for breast cancer with different radiotherapy techniques. Sci Rep 10(1):1220
Abo-Madyan Y, Aziz MH, Aly MM, Schneider F, Sperk E, Clausen S et al (2014) Second cancer risk after 3D-CRT, IMRT and VMAT for breast cancer. Radiother Oncol 110(3):471–476
Chera BS, Vargas C, Morris CG, Louis D, Flampouri S, Yeung D et al (2009) Dosimetric study of pelvic proton radiotherapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 75(4):994–1002
Fontenot JD, Lee AK, Newhauser WD (2009) Risk of secondary malignant neoplasms from proton therapy and intensity-modulated x-ray therapy for early-stage prostate cancer. Int J Radiat Oncol Biol Phys 74(2):616–622
Braunstein S, Nakamura JL (2013) Radiotherapy-induced malignancies: review of clinical features, pathobiology, and evolving approaches for mitigating risk. Front Oncol 3:73
Bougeard G, Renaux-Petel M, Flaman JM, Charbonnier C, Fermey P, Belotti M et al (2015) Revisiting li-fraumeni syndrome from TP53 mutation carriers. J Clin Oncol 33(21):2345–2352
Le AN, Harton J, Desai H, Powers J, Zelley K, Bradbury AR et al (2020) Frequency of radiation-induced malignancies post-adjuvant radiotherapy for breast cancer in patients with Li-Fraumeni syndrome. Breast Cancer Res Treat 181(1):181–188
Hendrickson PG, Luo Y, Kohlmann W, Schiffman J, Maese L, Bishop AJ et al (2020) Radiation therapy and secondary malignancy in Li-Fraumeni syndrome: a hereditary cancer registry study. Cancer Med 9(21):7954–7963
Wong FL, Boice JD Jr, Abramson DH, Tarone RE, Kleinerman RA, Stovall M et al (1997) Cancer incidence after retinoblastoma. Radiat Sarcoma Risk JAMA 278(15):1262–1267
Best T, Li D, Skol AD, Kirchhoff T, Jackson SA, Yasui Y et al (2011) Variants at 6q21 implicate PRDM1 in the etiology of therapy-induced second malignancies after Hodgkin’s lymphoma. Nat Med 17(8):941–943
Knight JA, Skol AD, Shinde A, Hastings D, Walgren RA, Shao J et al (2009) Genome-wide association study to identify novel loci associated with therapy-related myeloid leukemia susceptibility. Blood 113(22):5575–5582
Armstrong GT, Sklar CA, Hudson MM, Robison LL (2007) Long-term health status among survivors of childhood cancer: Does sex matter? J Clin Oncol 25(28):4477–4489
Bhatia S, Sklar C (2002) Second cancers in survivors of childhood cancer. Nat Rev Cancer 2(2):124–132
Prochazka M, Hall P, Gagliardi G, Granath F, Nilsson BN, Shields PG et al (2005) Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: case-only design. J Clin Oncol 23(30):7467–7474
Travis LB, Gospodarowicz M, Curtis RE, Clarke EA, Andersson M, Glimelius B et al (2002) Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst 94(3):182–192
Behjati S, Gundem G, Wedge DC, Roberts ND, Tarpey PS, Cooke SL et al (2016) Mutational signatures of ionizing radiation in second malignancies. Nat Commun 7:12605
Donson AM, Erwin NS, Kleinschmidt-DeMasters BK, Madden JR, Addo-Yobo SO, Foreman NK (2007) Unique molecular characteristics of radiation-induced glioblastoma. J Neuropathol Exp Neurol 66(8):740–749
Gonin-Laurent N, Gibaud A, Huygue M, Lefevre SH, Le Bras M, Chauveinc L et al (2006) Specific TP53 mutation pattern in radiation-induced sarcomas. Carcinogenesis 27(6):1266–1272
Manner J, Radlwimmer B, Hohenberger P, Mossinger K, Kuffer S, Sauer C et al (2010) MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol 176(1):34–39
Nguyen DH, Oketch-Rabah HA, Illa-Bochaca I, Geyer FC, Reis-Filho JS, Mao JH et al (2011) Radiation acts on the microenvironment to affect breast carcinogenesis by distinct mechanisms that decrease cancer latency and affect tumor type. Cancer Cell 19(5):640–651
Olschowka JA, Kyrkanides S, Harvey BK, O’Banion MK, Williams JP, Rubin P et al (1997) ICAM-1 induction in the mouse CNS following irradiation. Brain Behav Immun 11(4):273–285
Zhao Y, Zhang T, Wang Y, Lu D, Du J, Feng X et al (2021) ICAM-1 orchestrates the abscopal effect of tumor radiotherapy. Proc Natl Acad Sci 118(14):e2010333118
Chen B, Zhao Z, Lee V, Reddy R, Stoodley M (2016) Radiation-induced expression of platelet endothelial cell adhesion molecule-1 in cerebral endothelial cells. Int J Radiat Res 14(3):181–188
Sans E, Delachanal E, Duperray A (2001) Analysis of the roles of ICAM-1 in neutrophil transmigration using a reconstituted mammalian cell expression model: implication of ICAM-1 cytoplasmic domain and Rho-dependent signaling pathway. J Immunol 166(1):544–551
Williams MR, Luscinskas FW (2011) Leukocyte rolling and adhesion via ICAM-1 signals to endothelial permeability. Focus on “Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1.” Am J Physiol Cell Physiol 301(4):777–779
Grönloh MLB, Arts JJG, Martínez SP, van der Veen AA, Kempers L, van Steen ACI, et al. Endothelial transmigration hotspots limit vascular leakage through heterogeneous expression of ICAM1. bioRxiv. 2022:2022.01.14.476297
Padmanabhan J, Gonzalez AL (2012) The effects of extracellular matrix proteins on neutrophil-endothelial interaction–a roadway to multiple therapeutic opportunities. Yale J Biol Med 85(2):167–185
Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP (2005) Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol 81(12):887–899
Finkelstein JN, Johnston C, Barrett T, Oberdorster G (1997) Particulate-cell interactions and pulmonary cytokine expression. Environ Health Perspect 105(Suppl 5):1179–1182
Olman MA, White KE, Ware LB, Cross MT, Zhu S, Matthay MA (2002) Microarray analysis indicates that pulmonary edema fluid from patients with acute lung injury mediates inflammation, mitogen gene expression, and fibroblast proliferation through bioactive interleukin-1. Chest 121(3 Suppl):69S-70S
Porter DW, Ye J, Ma J, Barger M, Robinson VA, Ramsey D et al (2002) Time course of pulmonary response of rats to inhalation of crystalline silica: NF-kappa B activation, inflammation, cytokine production, and damage. Inhal Toxicol 14(4):349–367
Sedgwick JB, Menon I, Gern JE, Busse WW (2002) Effects of inflammatory cytokines on the permeability of human lung microvascular endothelial cell monolayers and differential eosinophil transmigration. J Allergy Clin Immunol 110(5):752–756
Di Maggio FM, Minafra L, Forte GI, Cammarata FP, Lio D, Messa C et al (2015) Portrait of inflammatory response to ionizing radiation treatment. J Inflamm (Lond) 12:14
Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32(5):593–604
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA (2006) Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24:99–146
Martin M, Lefaix J, Delanian S (2000) TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys 47(2):277–290
Anscher MS, Crocker IR, Jirtle RL (1990) Transforming growth factor-beta 1 expression in irradiated liver. Radiat Res 122(1):77–85
Barcellos-Hoff MH, Dix TA (1996) Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10(9):1077–1083
Barcellos-Hoff MH, Park C, Wright EG (2005) Radiation and the microenvironment-tumorigenesis and therapy. Nat Rev Cancer 5(11):867–875
Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S et al (2004) TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303(5659):848–851
Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA (1994) Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest 93(2):892–899
Wang J, Zheng H, Sung CC, Richter KK, Hauer-Jensen M (1998) Cellular sources of transforming growth factor-beta isoforms in early and chronic radiation enteropathy. Am J Pathol 153(5):1531–1540
Yarnold J, Brotons MC (2010) Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol 97(1):149–161
Chithra P, Sajithlal GB, Chandrakasan G (1998) Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. J Ethnopharmacol 59(3):179–186
Lefaix JL, Daburon F (1998) Diagnosis of acute localized irradiation lesions: review of the French experimental experience. Health Phys 75(4):375–384
Dracham CB, Shankar A, Madan R (2018) Radiation induced secondary malignancies: a review article. Radiat Oncol J 36(2):85–94
Khanna L, Prasad SR, Yedururi S, Parameswaran AM, Marcal LP, Sandrasegaran K et al (2021) Second malignancies after radiation therapy: update on pathogenesis and cross-sectional imaging findings. Radiographics 41(3):876–894
Nepon H, Safran T, Reece EM, Murphy AM, Vorstenbosch J, Davison PG (2021) Radiation-induced tissue damage: clinical consequences and current treatment options. Semin Plast Surg 35(3):181–188
Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM et al (2020) A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 20(3):174–186
Ejaz A, Greenberger JS, Rubin PJ (2019) Understanding the mechanism of radiation induced fibrosis and therapy options. Pharmacol Ther 204:107399
Yu G, Pang Y, Merchant M, Kesserwan C, Gangalapudi V, Abdelmaksoud A et al (2021) Tumor mutation burden, expressed neoantigens and the immune microenvironment in diffuse gliomas. Cancers (Basel). 13(23):6092
Kocakavuk E, Anderson KJ, Varn FS, Johnson KC, Amin SB, Sulman EP et al (2021) Radiotherapy is associated with a deletion signature that contributes to poor outcomes in patients with cancer. Nat Genet 53(7):1088–1096
Wang P, Chen Y, Wang C (2021) Beyond tumor mutation burden: tumor neoantigen burden as a biomarker for immunotherapy and other types of therapy. Front Oncol 11:672677
Lhuillier C, Rudqvist NP, Elemento O, Formenti SC, Demaria S (2019) Radiation therapy and anti-tumor immunity: exposing immunogenic mutations to the immune system. Genome Med 11(1):40
O’Sullivan T, Saddawi-Konefka R, Vermi W, Koebel CM, Arthur C, White JM et al (2012) Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med 209(10):1869–1882
Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ et al (2012) Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482(7385):400–404
Bao S, Jiang X, Jin S, Tu P, Lu J (2021) TGF-beta1 induces immune escape by enhancing PD-1 and CTLA-4 expression on T lymphocytes in hepatocellular carcinoma. Front Oncol 11:694145
Wang X, Yang L, Huang F, Zhang Q, Liu S, Ma L et al (2017) Inflammatory cytokines IL-17 and TNF-alpha up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol Lett 184:7–14
Lim SO, Li CW, Xia W, Cha JH, Chan LC, Wu Y et al (2016) Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell 30(6):925–939
Quandt D, Jasinski-Bergner S, Muller U, Schulze B, Seliger B (2014) Synergistic effects of IL-4 and TNFalpha on the induction of B7–H1 in renal cell carcinoma cells inhibiting allogeneic T cell proliferation. J Transl Med 12:151
Valero C, Lee M, Hoen D, Zehir A, Berger MF, Seshan VE et al (2021) Response rates to Anti-PD-1 immunotherapy in microsatellite-stable solid tumors with 10 or more mutations per megabase. JAMA Oncol 7(5):739–743
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520
Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M et al (2022) PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med 386(25):2363–2376
Solomon B, Young RJ, Bressel M, Urban D, Hendry S, Thai A et al (2018) Prognostic significance of PD-L1(+) and CD8(+) immune cells in HPV(+) oropharyngeal squamous cell carcinoma. Cancer Immunol Res 6(3):295–304
Briere D, Sudhakar N, Woods DM, Hallin J, Engstrom LD, Aranda R et al (2018) The class I/IV HDAC inhibitor mocetinostat increases tumor antigen presentation, decreases immune suppressive cell types and augments checkpoint inhibitor therapy. Cancer Immunol Immunother 67(3):381–392
Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R et al (2020) The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett 470:95–105
Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J et al (2019) EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol 30(8):1311–1320
Biton J, Mansuet-Lupo A, Pecuchet N, Alifano M, Ouakrim H, Arrondeau J et al (2018) TP53, STK11, and EGFR mutations predict tumor immune profile and the response to Anti-PD-1 in lung adenocarcinoma. Clin Cancer Res 24(22):5710–5723
Yi M, Qin S, Zhao W, Yu S, Chu Q, Wu K (2018) The role of neoantigen in immune checkpoint blockade therapy. Exp Hematol Oncol 7:28
Yi M, Li A, Zhou L, Chu Q, Luo S, Wu K (2021) Immune signature-based risk stratification and prediction of immune checkpoint inhibitor’s efficacy for lung adenocarcinoma. Cancer Immunol Immunother 70(6):1705–1719
Thompson JC, Hwang WT, Davis C, Deshpande C, Jeffries S, Rajpurohit Y et al (2020) Gene signatures of tumor inflammation and epithelial-to-mesenchymal transition (EMT) predict responses to immune checkpoint blockade in lung cancer with high accuracy. Lung Cancer 139:1–8
Author information
Authors and Affiliations
Contributions
T.A., M.R., D.K. and Z.M. conceptualize and wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Z. Morris declares that he is on the Scientific Advisory Board for Archeus Technologies and for Seneca Therapeutics. No disclosures were reported by the other authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Atajanova, T., Rahman, M.M., Konieczkowski, D.J. et al. Radiation-associated secondary malignancies: a novel opportunity for applying immunotherapies. Cancer Immunol Immunother 72, 3445–3452 (2023). https://doi.org/10.1007/s00262-023-03532-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03532-1