Abstract
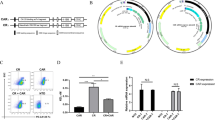
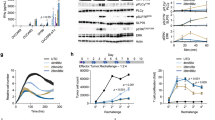
Epithelial ovarian cancer is the most lethal of gynecological cancers. The therapeutic efficacy of chimeric antigen receptor (CAR) T cell directed against single antigens is limited by the heterogeneous target antigen expression in epithelial ovarian tumors. To overcome this limitation, we describe an engineered cell with both dual targeting and orthogonal cytotoxic modalities directed against two tumor antigens that are highly expressed on ovarian cancer cells: cell surface Muc16 and intracellular WT1. Muc16-specific CAR T cells (4H11) were engineered to secrete a bispecific T cell engager (BiTE) constructed from a TCR mimic antibody (ESK1) reactive with the WT1-derived epitope RMFPNAPYL (RMF) presented by HLA-A2 molecules. The secreted ESK1 BiTE recruited and redirected other T cells to WT1 on the tumor cells. We show that ESK1 BiTE-secreting 4H11 CAR T cells exhibited enhanced anticancer activity against cancer cells with low Muc16 expression, compared to 4H11 CAR T cells alone, both in vitro and in mouse tumor models. Dual orthogonal cytotoxic modalities with different specificities targeting both surface and intracellular tumor-associated antigens present a promising strategy to overcome resistance to CAR T cell therapy in epithelial ovarian cancer and other cancers.






Similar content being viewed by others
References
Kroeger PT Jr, Drapkin R (2017) Pathogenesis and heterogeneity of ovarian cancer. Curr Opin Obstet Gynecol 29(1):26–34
Reid BM, Permuth JB, Sellers TA (2017) Epidemiology of ovarian cancer: a review. Cancer Biol Med 14(1):9–32
Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM (2018) Advances in ovarian cancer therapy. Cancer Chemother Pharmacol 81(1):17–38
Monk BJ, Choi DC, Pugmire G, Burger RA (2005) Activity of bevacizumab (rhuMAB VEGF) in advanced refractory epithelial ovarian cancer. Gynecol Oncol 96(3):902–905
Della Pepa C, Tonini G, Pisano C, Di Napoli M, Cecere SC, Tambaro R et al (2015) Ovarian cancer standard of care: are there real alternatives? Chin J Cancer 34(1):17–27
Sterner RC, Sterner RM (2021) CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 11(4):69
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H et al (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic Leukemia. N Engl J Med 378(5):439–448
Rafiq S, Hackett CS, Brentjens RJ (2020) Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 17(3):147–167
Kossaï M, Leary A, Scoazec JY, Genestie C (2018) Ovarian cancer: a heterogeneous disease. Pathobiology 85(1–2):41–49
Sarivalasis A, Morotti M, Mulvey A, Imbimbo M, Coukos G (2021) Cell therapies in ovarian cancer. Ther Adv Med Oncol 13:17588359211008400
Haas AR, Tanyi JL, O’Hara MH, Gladney WL, Lacey SF, Torigian DA et al (2019) Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther 27(11):1919–1929
Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ (2015) A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med 13:102
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348(3):203–213
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F et al (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 102(51):18538–18543
Goode EL, Block MS, Kalli KR, Vierkant RA, Chen W, Fogarty ZC et al (2017) Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol 3(12):e173290
Jones HF, Molvi Z, Klatt MG, Dao T, Scheinberg DA (2020) Empirical and rational design of T cell receptor-based immunotherapies. Front Immunol 11:585385
Oka Y, Tsuboi A, Kawakami M, Elisseeva OA, Nakajima H, Udaka K et al (2006) Development of WT1 peptide cancer vaccine against hematopoietic malignancies and solid cancers. Curr Med Chem 13(20):2345–2352
Anguille S, Willemen Y, Lion E, Smits EL, Berneman ZN (2012) Dendritic cell vaccination in acute myeloid leukemia. Cytotherapy 14(6):647–656
Chapuis AG, Egan DN, Bar M, Schmitt TM, McAfee MS, Paulson KG et al (2019) T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med 25(7):1064–1072
Chapuis AG, Ragnarsson GB, Nguyen HN, Chaney CN, Pufnock JS, Schmitt TM et al (2013) Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med 5(174):174ra27
Manning-Geist BL, Gnjatic S, Aghajanian C, Konner J, Kim SH, Sarasohn D et al (2023) Phase I study of a multivalent WT1 peptide vaccine (Galinpepimut-S) in combination with nivolumab in patients with WT1-expressing ovarian cancer in second or third remission. Cancers (Basel) 15(5):1458
Dao T, Pankov D, Scott A, Korontsvit T, Zakhaleva V, Xu Y et al (2015) Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat Biotechnol 33(10):1079–1086
Rafiq S, Purdon TJ, Daniyan AF, Koneru M, Dao T, Liu C et al (2017) Optimized T-cell receptor-mimic chimeric antigen receptor T cells directed toward the intracellular Wilms Tumor 1 antigen. Leukemia 31(8):1788–1797
Latouche JB, Sadelain M (2000) Induction of human cytotoxic T lymphocytes by artificial antigen-presenting cells. Nat Biotechnol 18(4):405–409
Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG et al (2013) CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5(177):177ra38
Chekmasova AA, Rao TD, Nikhamin Y, Park KJ, Levine DA, Spriggs DR et al (2010) Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res 16(14):3594–3606
Peraro L, Bourne CM, Dacek MM, Akalin E, Park JH, Smith EL et al (2021) Incorporation of bacterial immunoevasins to protect cell therapies from host antibody-mediated immune rejection. Mol Ther 29(12):3398–3409
Ataie N, Xiang J, Cheng N, Brea EJ, Lu W, Scheinberg DA et al (2016) Structure of a TCR-mimic antibody with target predicts pharmacogenetics. J Mol Biol 428(1):194–205
Gejman RS, Jones HF, Klatt MG, Chang AY, Oh CY, Chandran SS et al (2020) Identification of the targets of T-cell receptor therapeutic agents and cells by use of a high-throughput genetic platform. Cancer Immunol Res 8(5):672–684
Dharma Rao T, Park KJ, Smith-Jones P, Iasonos A, Linkov I, Soslow RA et al (2010) Novel monoclonal antibodies against the proximal (carboxy-terminal) portions of MUC16. Appl Immunohistochem Mol Morphol 18(5):462–472
Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ (2015) IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 4(3):e994446
Crawford A, Haber L, Kelly MP, Vazzana K, Canova L, Ram P et al (2019) A Mucin 16 bispecific T cell-engaging antibody for the treatment of ovarian cancer. Sci Transl Med. 11(497):eaau7534
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC (2018) CAR T cell immunotherapy for human cancer. Science 359(6382):1361–1365
Sugiyama H (2010) WT1 (Wilms’ tumor gene 1): biology and cancer immunotherapy. Jpn J Clin Oncol 40(5):377–387
Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC et al (2019) CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol 37(9):1049–1058
Choi BD, Gedeon PC, Herndon JE 2nd, Archer GE, Reap EA, Sanchez-Perez L et al (2013) Human regulatory T cells kill tumor cells through granzyme-dependent cytotoxicity upon retargeting with a bispecific antibody. Cancer Immunol Res 1(3):163
Funding
This work was supported by NIH RO1 CA55349, PO1 CA23766, P30 CA008748 and R35 CA241894 to DAS, and NCI 1R50CA265328-01A1 to TD. REO was funded in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748) and was funded in part by P30 CA008748.
Author information
Authors and Affiliations
Contributions
TD and DAS conceived the idea and wrote the manuscript. SSM, TG, LP, and TD co-designed the study, analyzed data, and participated in the manuscript preparation. SSM, JPM, LP, TK, and MM performed experiments. CL designed and provided the ESK1-BITE and the related materials. REO and CK provided clinical samples, expertise in ovarian cancer study, and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
DAS is on a board of, has equity in, receives research funding from, has licensed technology with, or income from Lantheus, Sellas, Iovance, Arvinas, Clade, Pfizer, Actinium, Oncopep, Repertoire, Sapience, Atengen, CoImmune, and Eureka Therapeutics. TD is a consultant of Eureka Therapeutics. REO reports personal fees from Tesaro/GSK, Regeneron, Seattle Genetics, Fresenius Kabi, Gynecologic Oncology Foundation, Bayer, Curio/Onclive, R-Pharm, and Immunogen, other from Hitech Health, outside the submitted work. She is a non-compensated steering committee member for the PRIMA, Moonstone (Tesaro/GSK) and DUO-O (AstraZeneca) studies and non-compensated advisor for Carina Biotech. Her institute receives funding for clinical research from Bayer/Celgene/Juno, Tesaro/GSK, Merck, Ludwig Cancer Institute, Abbvie/StemCentrx, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, MarkerTherapeutics, Syndax Pharmaceuticals, Genmab/Seagen Therapeutics, Sellas Therapeutics, Genentech, Kite Pharma, and Gynecologic Oncology Foundation. LP is now an employee of Beam. TG is now an employee of Arsenal. All other authors declare no conflict or competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Memorial Sloan Kettering Cancer Center Institutional Review Board (IRB Human Subject Assurance Number FW00004998) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mun, S.S., Meyerberg, J., Peraro, L. et al. Dual targeting ovarian cancer by Muc16 CAR T cells secreting a bispecific T cell engager antibody for an intracellular tumor antigen WT1. Cancer Immunol Immunother 72, 3773–3786 (2023). https://doi.org/10.1007/s00262-023-03529-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03529-w