Abstract
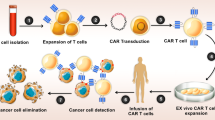
Triple negative breast cancer (TNBC) is a subtype of breast cancer with the highest degree of malignancy and the worst prognosis. The application of immunotherapy for TNBC is limited. This study was to verify the potential application of chimeric antigen receptor-T cells (CAR-T cells) targeting CD24 named as 24BBz in treatment of TNBC. 24BBz was constructed by lentivirus infection and then was co-culture with breast cancer cell lines to evaluate the activation, proliferation and cytotoxicity of engineered T cells. The anti-tumor activity of 24BBz was verified in the subcutaneous xenograft model of nude mice. We found that CD24 gene was significantly up-regulated in breast cancer (BRCA), especially in TNBC. 24BBz showed antigen-specific activation and dose-dependent cytotoxicity against CD24-positive BRCA tumor cells in vitro. Furthermore, 24BBz showed significant anti-tumor effect in CD24-positive TNBC xenografts and T cells infiltration in tumor tissues, while some T cells exhibited exhaustion. No pathological damage of major organs was found during the treatment. This study proved that CD24-specific CAR-T cells have potent anti-tumor activity and potential application value in treatment of TNBC.





Similar content being viewed by others
Data availability
Data are available on reasonable request.
References
Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G (2021) Breast cancer. Lancet (London, England) 397(10286):1750–1769. https://doi.org/10.1016/s0140-6736(20)32381-3
Bianchini G, De Angelis C, Licata L, Gianni L (2022) Treatment landscape of triple-negative breast cancer-expanded options, evolving needs. Nat Rev Clin Oncol 19(2):91–113. https://doi.org/10.1038/s41571-021-00565-2
Hua Z, White J, Zhou J (2022) Cancer stem cells in TNBC. Semin Cancer Biol 82:26–34. https://doi.org/10.1016/j.semcancer.2021.06.015
Garrido-Castro AC, Lin NU, Polyak K (2019) Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov 9(2):176–198. https://doi.org/10.1158/2159-8290.Cd-18-1177
He MY et al (2018) Radiotherapy in triple-negative breast cancer: current situation and upcoming strategies. Crit Rev Oncol Hematol 131:96–101. https://doi.org/10.1016/j.critrevonc.2018.09.004
Nedeljković M, Damjanović A (2019) Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells. https://doi.org/10.3390/cells8090957
Jabbarzadeh Kaboli P et al (2022) Shedding light on triple-negative breast cancer with Trop2-targeted antibody-drug conjugates. Am J Cancer Res 12(4):1671–1685
Kristiansen G et al (2003) CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res 9(13):4906–4913
Pirruccello SJ, LeBien TW (1986) The human B cell-associated antigen CD24 is a single chain sialoglycoprotein. J Immunol (Baltimore, md.: 1950) 136(10):3779–3784
Tarhriz V et al (2019) Overview of CD24 as a new molecular marker in ovarian cancer. J Cell Physiol 234(3):2134–2142. https://doi.org/10.1002/jcp.27581
Barkal AA et al (2019) CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 572(7769):392–396. https://doi.org/10.1038/s41586-019-1456-0
Baumann P et al (2012) CD24 interacts with and promotes the activity of c-src within lipid rafts in breast cancer cells, thereby increasing integrin-dependent adhesion. Cell Mol Life Sci 69(3):435–448. https://doi.org/10.1007/s00018-011-0756-9
Kwon MJ et al (2015) CD24 overexpression is associated with poor prognosis in luminal a and triple-negative breast cancer. PLoS ONE 10(10):e0139112. https://doi.org/10.1371/journal.pone.0139112
Chan SH et al (2019) Identification of the novel role of CD24 as an oncogenesis regulator and therapeutic target for triple-negative breast cancer. Mol Cancer Ther 18(1):147–161. https://doi.org/10.1158/1535-7163.Mct-18-0292
Deng X et al (2017) CD24 Expression and differential resistance to chemotherapy in triple-negative breast cancer. Oncotarget 8(24):38294–38308. https://doi.org/10.18632/oncotarget.16203
Jing X et al (2018) CD24 is a potential biomarker for prognosis in human breast carcinoma. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 48(1):111–119. https://doi.org/10.1159/000491667
Maliar A et al (2012) Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 143(5):1375-1384.e1375. https://doi.org/10.1053/j.gastro.2012.07.017
Sun F et al (2021) Bispecific CAR-T cells targeting both BCMA and CD24: a potentially treatment approach for multiple myeloma. Blood 138:2802
Sun F et al (2017) Engineering a high-affinity humanized anti-CD24 antibody to target hepatocellular carcinoma by a novel CDR grafting design. Oncotarget 8(31):51238–51252. https://doi.org/10.18632/oncotarget.17228
Jayaraman J et al (2020) CAR-T design: elements and their synergistic function. EBioMedicine 58:102931. https://doi.org/10.1016/j.ebiom.2020.102931
Xu N et al (2021) STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J Exp Med. https://doi.org/10.1084/jem.20200844
Liedtke C et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26(8):1275–1281. https://doi.org/10.1200/jco.2007.14.4147
Zhao Z, Chen Y, Francisco NM, Zhang Y, Wu M (2018) The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B 8(4):539–551. https://doi.org/10.1016/j.apsb.2018.03.001
Xia L et al (2020) EGFR-targeted CAR-T cells are potent and specific in suppressing triple-negative breast cancer both in vitro and in vivo. Clin Transl Immunol 9(5):e01135. https://doi.org/10.1002/cti2.1135
Corti C et al (2022) CAR-T cell therapy for triple-negative breast cancer and other solid tumors: preclinical and clinical progress. Expert Opin Investig Drugs 31(6):593–605. https://doi.org/10.1080/13543784.2022.2054326
Tchou J et al (2017) Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol Res 5(12):1152–1161. https://doi.org/10.1158/2326-6066.Cir-17-0189
Yamashita N et al (2021) MUC1-C integrates activation of the IFN-γ pathway with suppression of the tumor. J Immunother Cancer 9:1
Wei J et al (2018) A novel AXL chimeric antigen receptor endows T cells with anti-tumor effects. Cell Immunol 331:49–58
Chen H et al (2021) CD27 enhances the killing effect of CAR T cells targeting trophoblast cell. Cancer Immunol Immunother 70:2059–2071
Yang XR et al (2009) CD24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clin Cancer Res 15(17):5518–5527. https://doi.org/10.1158/1078-0432.Ccr-09-0151
He H et al (2015) A novel antibody targeting CD24 and hepatocellular carcinoma in vivo by near-infrared fluorescence imaging. Immunobiology 220(12):1328–1336. https://doi.org/10.1016/j.imbio.2015.07.010
Maus MV et al (2013) T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res 1:26–31
Lv J et al (2019) Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer. J Hematol Oncol 12(1):18. https://doi.org/10.1186/s13045-019-0704-y
Cherkassky L, Hou Z, Amador-Molina A, Adusumilli PS (2022) Regional CAR T cell therapy: an ignition key for systemic immunity in solid tumors. Cancer Cell 40(6):569–574. https://doi.org/10.1016/j.ccell.2022.04.006
Adusumilli PS et al (2014) Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 6(261):261151. https://doi.org/10.1126/scitranslmed.3010162
Hou AJ, Chen LC, Chen YY (2021) Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov 20(7):531–550. https://doi.org/10.1038/s41573-021-00189-2
Sun C, Mezzadra R, Schumacher TN (2018) Regulation and function of the PD-L1 checkpoint. Immunity 48(3):434–452. https://doi.org/10.1016/j.immuni.2018.03.014
Cherkassky L et al (2016) Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 126(8):3130–3144. https://doi.org/10.1172/jci83092
Grosser R, Cherkassky L, Chintala N, Adusumilli PS (2019) Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 36(5):471–482. https://doi.org/10.1016/j.ccell.2019.09.006
Rafiq S et al (2018) Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 36(9):847–856. https://doi.org/10.1038/nbt.4195
Choi BD et al (2019) CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer 7(1):304. https://doi.org/10.1186/s40425-019-0806-7
Yu S, Yi M, Qin S, Wu K (2019) Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer 18(1):125. https://doi.org/10.1186/s12943-019-1057-4
Rafiq S, Hackett CS, Brentjens RJ (2020) Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 17(3):147–167. https://doi.org/10.1038/s41571-019-0297-y
Paszkiewicz PJ et al (2016) Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest 126(11):4262–4272. https://doi.org/10.1172/jci84813
Funding
This work was supported by the Project Program of State Key Laboratory of Natural Medicines (SKLNMZZ202219), the National Natural Science Foundation (NSFC81973223), the Fundamental Research Funds for the Central Universities (2632023GR08) and the Nanjing International Industry Technology Research and Development Cooperation Project (2021SX00000435-202100880).
Author information
Authors and Affiliations
Contributions
YPW, YF and YZ designed and performed the experiments, analyzed the data, created the figures and wrote the paper. DX collected the blood sample. ZJ and XHM conceived the idea, supervised the project, and edited the paper. All authors carefully read and approved the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Animal experiments were conducted in conformity with guidelines of the China Pharmaceutical University.
Consent to participate
Fresh blood was obtained from healthy volunteers with the approval of the Ethics Committee of China Pharmaceutical University. Informed consent was obtained from all individual participants.
Consent to publish
All authors and subjects in this study concur with the publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, P., Yu, F., Yao, Z. et al. CD24 is a novel target of chimeric antigen receptor T cells for the treatment of triple negative breast cancer. Cancer Immunol Immunother 72, 3191–3202 (2023). https://doi.org/10.1007/s00262-023-03491-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03491-7