Abstract
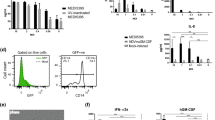
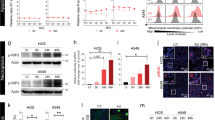
Antitumor virotherapy stimulates the antitumor immune response during tumor cell lysis induced by oncolytic viruses (OVs). OV can be modified to express additional transgenes that enhance their therapeutic potential. In this study, we armed the spontaneously oncolytic Schwarz strain of measles viruses (MVs) with the gene encoding the cancer/testis antigen NY-ESO-1 to obtain MVny. We compared MV and MVny oncolytic activity and ability to induce NY-ESO-1 expression in six human melanoma cell lines. After MVny infection, we measured the capacity of melanoma cells to present NY-ESO-1 peptides to CD4 + and CD8 + T cell clones specific for this antigen. We assessed the ability of MVny to induce NY-ESO-1 expression and presentation in monocyte-derived dendritic cells (DCs). Our results show that MVny and MV oncolytic activity are similar with a faster cell lysis induced by MVny. We also observed that melanoma cell lines and DC expressed the NY-ESO-1 protein after MVny infection. In addition, MVny-infected melanoma cells and DCs were able to stimulate NY-ESO-1-specific CD4 + and CD8 + T cells. Finally, MVny was able to induce DC maturation. Altogether, these results show that MVny could be an interesting candidate to stimulate NY-ESO-1-specific T cells in melanoma patients with NY-ESO-1-expressing tumor cells.








Similar content being viewed by others
Data availability statement
The data that support the findings of this study are available from the corresponding author (jean-francois.fonteneau@inserm.fr) upon reasonable request.
References
Macedo N, Miller DM, Haq R, Kaufman HL (2020) Clinical landscape of oncolytic virus research in 2020. J Immunother Cancer 8
Achard C, Surendran A, Wedge ME et al (2018) Lighting a fire in the tumor microenvironment using oncolytic immunotherapy. EBioMedicine 31:17–24
Gauvrit A, Brandler S, Sapede-Peroz C et al (2008) Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res 68:4882–4892
Guillerme JB, Boisgerault N, Roulois D et al (2013) Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin Cancer Res 19:1147–1158
Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T (2014) Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 14:135–146
Meng X, Sun X, Liu Z, He Y (2021) A novel era of cancer/testis antigen in cancer immunotherapy. Int Immunopharmacol 98:107889
Gnjatic S, Nishikawa H, Jungbluth AA et al (2006) NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res 95:1–30
Jager E, Chen YT, Drijfhout JW et al (1998) Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med 187:265–270
Wang RF, Wang HY (2017) Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res 27:11–37
Thomas R, Al-Khadairi G, Roelands J et al (2018) NY-ESO-1 based immunotherapy of cancer: current perspectives. Front Immunol 9:947
Allagui F, Achard C, Panterne C et al (2017) Modulation of the type I interferon response defines the sensitivity of human melanoma cells to oncolytic measles virus. Curr Gene Ther 16:419–428. https://doi.org/10.2174/1566523217666170102110502
Boisgerault N, Guillerme JB, Pouliquen D et al (2013) Natural oncolytic activity of live-attenuated measles virus against human lung and colorectal adenocarcinomas. Biomed Res Int 2013:387362
Achard C, Boisgerault N, Delaunay T et al (2015) Sensitivity of human pleural mesothelioma to oncolytic measles virus depends on defects of the type I interferon response. Oncotarget 6:44892–44904. https://doi.org/10.18632/oncotarget.6285
Delaunay T, Achard C, Boisgerault N et al (2020) Frequent homozygous deletions of type i interferon genes in pleural mesothelioma confer sensitivity to oncolytic measles virus. J Thoracic Oncol : Official Public Int Assoc Study of Lung Cancer 15:827–842. https://doi.org/10.1016/j.jtho.2019.12.128
Msaouel P, Opyrchal M, Dispenzieri A et al (2018) Clinical trials with oncolytic measles virus: current status and future prospects. Curr Cancer Drug Targets 18:177–187
Backhaus PS, Veinalde R, Hartmann L, et al (2019) Immunological effects and viral gene expression determine the efficacy of oncolytic measles vaccines encoding IL-12 or IL-15 Agonists. Viruses 11
Speck T, Heidbuechel JPW, Veinalde R et al (2018) Targeted BiTE expression by an oncolytic vector augments therapeutic efficacy against solid tumors. Clin Cancer Res 24:2128–2137
Dispenzieri A, Tong C, LaPlant B et al (2017) Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma. Leukemia 31:2791–2798
Frantz PN, Barinov A, Ruffie C et al (2021) A live measles-vectored COVID-19 vaccine induces strong immunity and protection from SARS-CoV-2 challenge in mice and hamsters. Nat Commun 12:6277
Ramsauer K, Schwameis M, Firbas C et al (2015) Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect Dis 15:519–527
Pol JG, Zhang L, Bridle BW et al (2014) Maraba virus as a potent oncolytic vaccine vector. Mol Ther 22:420–429. https://doi.org/10.1038/mt.2013.249
Busch E, Kubon KD, Mayer JKM et al (2020) Measles vaccines designed for enhanced CD8+ T cell activation. Viruses 12:242. https://doi.org/10.3390/v12020242
Fonteneau JF, Larsson M, Somersan S et al (2001) Generation of high quantities of viral and tumor-specific human CD4+ and CD8+ T-cell clones using peptide pulsed mature dendritic cells. J Immunol Methods 258:111–126
Roulois D, Vignard V, Gueugnon F et al (2011) Recognition of pleural mesothelioma by mucin-1(950–958)/human leukocyte antigen A*0201-specific CD8+ T-cells. Eur Respir J 38:1117–1126
Benlalam H, Labarrière N, Linard B et al (2001) Comprehensive analysis of the frequency of recognition of melanoma-associated antigen (MAA) by CD8 melanoma infiltrating lymphocytes (TIL): implications for immunotherapy. Eur J Immunol 31:2007–2015. https://doi.org/10.1002/1521-4141(200107)31:7%3c2007::aid-immu2007%3e3.0.co;2-s
Coulais D, Panterne C, Fonteneau JF, Gregoire M (2012) Purification of circulating plasmacytoid dendritic cells using counterflow centrifugal elutriation and immunomagnetic beads. Cytotherapy 14:887–896
Combredet C, Labrousse V, Mollet L et al (2003) A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J Virol 77:11546–11554
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. https://doi.org/10.1373/clinchem.2008.112797
Ohgimoto K, Ohgimoto S, Ihara T et al (2007) Difference in production of infectious wild-type measles and vaccine viruses in monocyte-derived dendritic cells. Virus Res 123:1–8. https://doi.org/10.1016/j.virusres.2006.07.006
Sanchez David RY, Combredet C, Sismeiro O et al (2016) Comparative analysis of viral RNA signatures on different RIG-I-like receptors. eLife 5: 11275
Mura M, Combredet C, Najburg V et al (2017) Nonencapsidated 5′ copy-back defective interfering genomes produced by recombinant measles viruses are recognized by RIG-I and LGP2 but not MDA5. J Virol 91:e00643-e717. https://doi.org/10.1128/JVI.00643-17
Donnelly OG, Errington-Mais F, Steele L et al (2011) Measles virus causes immunogenic cell death in human melanoma. Gene Ther
Fonteneau JF, Brilot F, Munz C, Gannage M (2016) The tumor antigen NY-ESO-1 mediates direct recognition of melanoma cells by CD4+ T cells after intercellular antigen transfer. J Immunol 196:64–71
Delaunay T, Violland M, Boisgerault N et al (2018) Oncolytic viruses sensitize human tumor cells for NY-ESO-1 tumor antigen recognition by CD4+ effector T cells. Oncoimmunology 7:e1407897. https://doi.org/10.1080/2162402x.2017.1407897
Velazquez EF, Jungbluth AA, Yancovitz M et al (2007) Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)–correlation with prognostic factors. Cancer Immun 7:11
Barrow C, Browning J, MacGregor D et al (2006) Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res 12:764–771
Goydos JS, Patel M, Shih W (2001) NY-ESO-1 and CTp11 expression may correlate with stage of progression in melanoma. J Surg Res 98:76–80
Hunder NN, Wallen H, Cao J et al (2008) Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 358:2698–2703
Robbins PF, Kassim SH, Tran TL et al (2015) A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 21:1019–1027
Acknowledgements
We thank Philippe Hulin and the cellular and tissular core facility of Nantes University (MicroPiCell) for their expertise in video microscopy. We thank Nicolas Jouand and the core facility of flow cytometry from Nantes University (Cytocell).
Funding
This work was supported by “La Ligue Régionale Grand Ouest contre le Cancer” (CD16, CD22, CD44, CD49, CD72, CD79 and CD85), “l’association ARSMESO44”, “la Fondation ARC (PJA 20191209661)”, “l’Agence Nationale pour la Recherche (ANR-16-CE18-0016)”, and “LabEX IGO” program supported by the National Research Agency via the investment of the future program ANR-11-LABX-0016–01”.
Author information
Authors and Affiliations
Contributions
FT and JFF contributed to experiments conception. MG, MI, AA, CCh, LB, CCo, SD, VD, NB and JFF contributed to experiments. MG, NL, DF, NB, CB, FT and JFF performed data analysis and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
FT and JFF are authors of patents on MV. FT owns equity in Oncovita, an oncolytic virotherapy company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grard, M., Idjellidaine, M., Arbabian, A. et al. Oncolytic attenuated measles virus encoding NY-ESO-1 induces HLA I and II presentation of this tumor antigen by melanoma and dendritic cells. Cancer Immunol Immunother 72, 3309–3322 (2023). https://doi.org/10.1007/s00262-023-03486-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03486-4