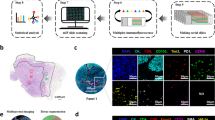
Abstract
Purpose
To explore the relationship between the spatial interaction of programmed death-ligand 1(PD-L1)-positive tumor cell and T cell with specific functions and the recurrence of non-small cell lung cancer (NSCLC) and optimize prognostic stratification.
Materials and methods
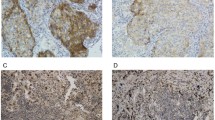
This study retrospectively included 104 patients with locally advanced NSCLC who underwent radical surgery. Tissue microarrays were constructed including tumor center (TC) and invasion margin (IM), and CK/CD4/CD8/PD-L1/programmed death-1 (PD-1) was labeled using multiplex immunofluorescence to decipher the counts and spatial distribution of tumor cells and T cells. The immune microenvironment and recurrence stratification were characterized using the Mann–Whitney U test and Cox proportional hazards model.
Result
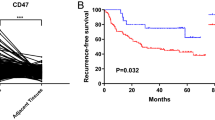
Compared with the IM, the proportion of tumor cells (especially PD-L1+) was increased in the TC, while T cells (especially PD-1+) were decreased. An increase in TC PD-1+ CD8 T cells promoted relapse (HR = 2.183), while PD-L1+ tumor cells alone or in combination with T cells had no predictive value for relapse. In addition, in both TC and IM, CD8 were on average closer to PD-L1+ tumor cells than CD4, especially exhausted CD8. The effective density and percentage of PD-1+ CD4 T cells interacting with PD-L1+ tumor cells in the IM were both associated with recurrence, and the HRs increased sequentially (HRs were 2.809 and 4.063, respectively). Patients with low PD-1+CD4 count combined high PD-1+CD4 effective density showed significantly poorer RFS compared to those with high PD-1+CD4 count combined low PD-1+CD4 effective density, in both the TC and IM regions (HRs were 5.810 and 8.709, respectively).
Conclusion
Assessing the relative spatial proximity of PD-1/PD-L1 contributes to a deeper understanding of tumor immune escape and generates prognostic information in locally advanced NSCLC patients.







Similar content being viewed by others
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Abbreviations
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- EGFR:
-
Epidermal growth factor receptor
- FFPE:
-
Formalin-fixed, paraffin-embedded
- IM:
-
Invasion margin
- ICIs:
-
Immune checkpoint inhibitors
- mIF:
-
Multiplex immunofluorescence
- NSCLC:
-
Non-small cell lung cancer
- NND:
-
Nearest neighbor distances
- PD-L1:
-
Programmed death-ligand 1
- PD-1:
-
Programmed death-1
- RFS:
-
Relapse-free survival
- TME:
-
Tumor microenvironment
- TMA:
-
Tissue microarray
- TC:
-
Tumor center
References
Galon J, Mlecnik B, Bindea G et al (2014) Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 232:199–209. https://doi.org/10.1002/path.4287
Wang D, DuBois RN (2015) Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 36:1085–1093. https://doi.org/10.1093/carcin/bgv123
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. https://doi.org/10.1038/nrc3239
Balança C-C, Salvioni A, Scarlata C-M et al (2021) PD-1 blockade restores helper activity of tumor-infiltrating, exhausted PD-1hiCD39+ CD4 T cells. JCI Insight 6:142513. https://doi.org/10.1172/jci.insight.142513
Zhou F, Qiao M, Zhou C (2021) The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell Mol Immunol 18:279–293. https://doi.org/10.1038/s41423-020-00577-5
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355. https://doi.org/10.1126/science.aar4060
Topalian SL (2017) Targeting immune checkpoints in cancer therapy. JAMA 318:1647. https://doi.org/10.1001/jama.2017.14155
Zolkind P, Uppaluri R (2017) Checkpoint immunotherapy in head and neck cancers. Cancer Metastasis Rev 36:475–489. https://doi.org/10.1007/s10555-017-9694-9
Barata PC, Rini BI (2017) Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin 67:507–524. https://doi.org/10.3322/caac.21411
Forde PM, Spicer J, Lu S et al (2022) Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 386:1973–1985. https://doi.org/10.1056/NEJMoa2202170
Spigel DR, Faivre-Finn C, Gray JE et al (2022) Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small cell lung cancer. J Clin Oncol 40:1301–1311. https://doi.org/10.1200/JCO.21.01308
Ahn H, Lee HJ, Lee J-H, et al (2020) Clinicopathological correlation of PD-L1 and TET1 expression with tumor-infiltrating lymphocytes in non-small cell lung cancer. Pathol Res Pract 216:153188. https://doi.org/10.1016/j.prp.2020.153188
Meng X, Gao Y, Yang L et al (2018) Immune microenvironment differences between squamous and non-squamous non–small-cell lung cancer and their influence on the prognosis. Clin Lung Cancer 20:48–58. https://doi.org/10.1016/j.cllc.2018.09.012
Chen L, Cao M, Zhang X et al (2019) The landscape of immune microenvironment in lung adenocarcinoma and squamous cell carcinoma based on PD-L1 expression and tumor-infiltrating lymphocytes. Cancer Med 8:7207–7218. https://doi.org/10.1002/cam4.2580
Edlund K, Madjar K, Mattsson JSM et al (2019) Prognostic impact of tumor cell programmed death ligand 1 expression and immune cell infiltration in NSCLC. J Thorac Oncol 14:628–640. https://doi.org/10.1016/j.jtho.2018.12.022
Zheng H, Ning Y, Zhan Y et al (2021) Co-expression of PD-L1 and HIF-1α predicts poor prognosis in patients with non-small cell lung cancer after surgery. J Cancer 12:2065–2072. https://doi.org/10.7150/jca.53119
Cao L, Wang X, Li S et al (2017) PD-L1 is a prognostic biomarker in resected NSCLC patients with moderate/high smoking history and elevated serum SCCA level. J Cancer 8:3251–3260. https://doi.org/10.7150/jca.21118
Yang H, Shi J, Lin D et al (2017) Prognostic value of PD-L1 expression in combination with CD8+TILs density in patients with surgically resected non-small cell lung cancer. Cancer Med 7:32–45. https://doi.org/10.1002/cam4.1243
Handa Y, Tsutani Y, Shiroma N et al (2020) Prognostic impact of programmed death-ligand 1 and surrounding immune status on stage I lung cancer. Clin Lung Cancer 21:e302–e314. https://doi.org/10.1016/j.cllc.2020.01.013
Guo X, Zhang Y, Zheng L et al (2018) Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med 24:978–985. https://doi.org/10.1038/s41591-018-0045-3
Lhuillier C, Rudqvist N-P, Yamazaki T et al (2021) Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J Clin Invest 131:138740. https://doi.org/10.1172/JCI138740
Tan WCC, Nerurkar SN, Cai HY et al (2020) Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun (Lond) 40:135–153. https://doi.org/10.1002/cac2.12023
Väyrynen SA, Zhang J, Yuan C et al (2021) Composition, spatial characteristics, and prognostic significance of myeloid cell infiltration in pancreatic cancer. Clin Cancer Res 27(4):1069–1081. https://doi.org/10.1158/1078-0432.CCR-20-3141
Tsakiroglou AM, Fergie M, Oguejiofor K et al (2020) Spatial proximity between T and PD-L1 expressing cells as a prognostic biomarker for oropharyngeal squamous cell carcinoma. Br J Cancer 122:539–544. https://doi.org/10.1038/s41416-019-0634-z
Zheng X, Weigert A, Reu S et al (2020) Spatial density and distribution of tumor-associated macrophages predict survival in non–small cell lung carcinoma. Cancer Res 80:4414–4425. https://doi.org/10.1158/0008-5472.CAN-20-0069
Pøhl M, Olsen KE, Holst R et al (2014) Tissue microarrays in non–small-cell lung cancer: reliability of immunohistochemically-determined biomarkers. Clin Lung Cancer 15:222-230.e3. https://doi.org/10.1016/j.cllc.2013.09.004
Schalper KA, Brown J, Carvajal-Hausdorf D, et al (2015) Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst 107(3):dju435. https://doi.org/10.1093/jnci/dju435
Donnem T, Hald SM, Paulsen E-E et al (2015) Stromal CD8+ T-cell Density—a promising supplement to TNM Staging in non–small cell lung cancer. Clin Cancer Res 21:2635–2643. https://doi.org/10.1158/1078-0432.CCR-14-1905
Mlecnik B, Bindea G, Kirilovsky A et al (2016) The tumor microenvironment and immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med 24;8(327):327ra26. https://doi.org/10.1126/scitranslmed.aad6352
Parra ER, Uraoka N, Jiang M et al (2017) Validation of multiplex immunofluorescence panels using multispectral microscopy for immune-profiling of formalin-fixed and paraffin-embedded human tumor tissues. Sci Rep 17;7(1):13380. https://doi.org/10.1038/s41598-017-13942-8
Ying L, Yan F, Meng Q et al (2018) PD-L1 expression is a prognostic factor in subgroups of gastric cancer patients stratified according to their levels of CD8 and FOXP3 immune markers. Oncoimmunology 20;7(6):e1433520. https://doi.org/10.1080/2162402X.2018.1433520
Zheng B, Wang D, Qiu X et al (2020) Trajectory and functional analysis of PD-1highCD4+CD8+T Cells in hepatocellular carcinoma by single-cell cytometry and transcriptome sequencing. Adv Sci 7:2000224. https://doi.org/10.1002/advs.202000224
Bastola S, Pavlyukov MS, Yamashita D et al (2020) Glioma-initiating cells at tumor edge gain signals from tumor core cells to promote their malignancy. Nat Commun 11:4660. https://doi.org/10.1038/s41467-020-18189-y
van der Leun AM, Thommen DS, Schumacher TN (2020) CD8+ T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer 20:218–232. https://doi.org/10.1038/s41568-019-0235-4
Johnson DB, Bordeaux J, Kim JY et al (2018) Quantitative spatial profiling of PD-1/PD-L1 interaction and HLA-DR/IDO-1 predicts improved outcomes of anti–PD-1 therapies in metastatic melanoma. Clin Cancer Res 24:5250–5260. https://doi.org/10.1158/1078-0432.CCR-18-0309
Alspach E, Lussier DM, Miceli AP et al (2019) MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 574:696–701. https://doi.org/10.1038/s41586-019-1671-8
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82172866), the Shandong Provincial Natural Science Foundation (Grant No. ZR2021LZL005), the Shandong Provincial Natural Science Foundation (Grant No. ZR2019LZL019), and the Department of Science & Technology of Shandong Province (Grant No. 2021CXGC011102).
Author information
Authors and Affiliations
Contributions
LY, LX, and XS contributed to conception and design of the study. LY, WZ, JS, GY, SC, and FS organized the database. LY, WZ, JS, GY, and SC performed the statistical analysis. LY wrote the first draft of the manuscript. LX and XS wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the Ethics Review Committee of Shandong Cancer Hospital and complied with the provisions of the Declaration of Helsinki. This study was a retrospective analysis, and informed consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, L., Zhang, W., Sun, J. et al. The role of spatial interplay patterns between PD-L1-positive tumor cell and T cell in recurrence of locally advanced non-small cell lung cancer. Cancer Immunol Immunother 72, 2015–2027 (2023). https://doi.org/10.1007/s00262-023-03380-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03380-z