Abstract
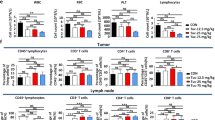
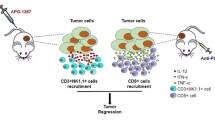
Pancreatic ductal adenocarcinoma (PDAC) is often refractory to treatment with gemcitabine (GEM) and immune checkpoint inhibitors including anti-programmed cell death ligand 1 (PD-L1) antibody. However, the precise relationship between GEM-resistant PDAC and development of an immunosuppressive tumor microenvironment (TME) remains unclear. In this study, we investigated the immunosuppressive TME in parental and GEM-resistant PDAC tumors and assessed the therapeutic potential of combination therapy with the telomerase-specific replication-competent oncolytic adenovirus OBP-702, which induces tumor suppressor p53 protein and PD-L1 blockade against GEM-resistant PDAC tumors. Mouse PDAC cells (PAN02) and human PDAC cells (MIA PaCa-2, BxPC-3) were used to establish GEM-resistant PDAC lines. PD-L1 expression and the immunosuppressive TME were analyzed using parental and GEM-resistant PDAC cells. A cytokine array was used to investigate the underlying mechanism of immunosuppressive TME induction by GEM-resistant PAN02 cells. The GEM-resistant PAN02 tumor model was used to evaluate the antitumor effect of combination therapy with OBP-702 and PD-L1 blockade. GEM-resistant PDAC cells exhibited higher PD-L1 expression and produced higher granulocyte–macrophage colony-stimulating factor (GM-CSF) levels compared with parental cells, inducing an immunosuppressive TME and the accumulation of myeloid-derived suppressor cells (MDSCs). OBP-702 significantly inhibited GEM-resistant PAN02 tumor growth by suppressing GM-CSF-mediated MDSC accumulation. Moreover, combination treatment with OBP-702 significantly enhanced the antitumor efficacy of PD-L1 blockade against GEM-resistant PAN02 tumors. The present results suggest that combination therapy involving OBP-702 and PD-L1 blockade is a promising antitumor strategy for treating GEM-resistant PDAC with GM-CSF-induced immunosuppressive TME formation.






Similar content being viewed by others
Abbreviations
- BMDC:
-
Bone marrow-derived cell
- CFSE:
-
Carboxyfluorescein diacetate succinimidyl ester
- CM:
-
Conditioned medium
- CTL:
-
Cytotoxic T lymphocyte
- ELISA:
-
Enzyme-linked immunosorbent assay
- FBS:
-
Fetal bovine serum
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GEM:
-
Gemcitabine
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- hTERT:
-
Human telomerase reverse transcriptase
- ICI:
-
Immune checkpoint inhibitor
- ICD:
-
Immunogenic cell death
- IC50 :
-
50% Inhibitory concentration
- MDSC:
-
Myeloid-derived suppressor cell
- MOI:
-
Multiplicity of infection
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PD-L1:
-
Programmed cell death ligand 1
- PD-1:
-
Programmed cell death 1
- PFU:
-
Plaque-forming unit
- TME:
-
Tumor microenvironment
- Treg:
-
Regulatory T cell
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74:2913–2921. https://doi.org/10.1158/0008-5472.CAN-14-0155
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer Statistics, 2021. CA Cancer J Clin 71:7–33. https://doi.org/10.3322/caac.21654
Burris HA 3rd, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413. https://doi.org/10.1200/JCO.1997.15.6.2403
Gu Z, Du Y, Zhao X, Wang C (2021) Tumor microenvironment and metabolic remodeling in gemcitabine-based chemoresistance of pancreatic cancer. Cancer Lett 521:98–108. https://doi.org/10.1016/j.canlet.2021.08.029
Sun C, Mezzadra R, Schumacher TN (2018) Regulation and function of the PD-L1 checkpoint. Immunity 48:434–452. https://doi.org/10.1016/j.immuni.2018.03.014
Leinwand J, Miller G (2020) Regulation and modulation of antitumor immunity in pancreatic cancer. Nat Immunol 21:1152–1159. https://doi.org/10.1038/s41590-020-0761-y
Herbst RS, Soria JC, Kowanetz M et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–567. https://doi.org/10.1038/nature14011
Yarchoan M, Albacker LA, Hopkins AC et al (2019) PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 4:e126908. https://doi.org/10.1172/jci.insight.126908
Gorchs L, Kaipe H (2021) Interactions between cancer-associated fibroblasts and T cells in the pancreatic tumor microenvironment and the role of chemokines. Cancers (Basel) 13:2995. https://doi.org/10.3390/cancers13122995
Blando J, Sharma A, Higa MG et al (2019) Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc Natl Acad Sci USA 116:1692–1697. https://doi.org/10.1073/pnas.1811067116
Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM, Greenberg PD, Hingorani SR (2014) Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 63:1769–1781. https://doi.org/10.1136/gutjnl-2013-306271
Candido JB, Morton JP, Bailey P et al (2018) CSF1R(+) macrophages sustain pancreatic tumor growth through T cell suppression and maintenance of key gene programs that define the squamous subtype. Cell Rep 23:1448–1460. https://doi.org/10.1016/j.celrep.2018.03.131
Viehl CT, Moore TT, Liyanage UK, Frey DM, Ehlers JP, Eberlein TJ, Goedegebuure PS, Linehan DC (2006) Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann Surg Oncol 13:1252–1258. https://doi.org/10.1245/s10434-006-9015-y
Doi T, Ishikawa T, Okayama T et al (2017) The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol Rep 37:545–554. https://doi.org/10.3892/or.2017.5399
Takeuchi S, Baghdadi M, Tsuchikawa T et al (2015) Chemotherapy-derived inflammatory responses accelerate the formation of immunosuppressive myeloid cells in the tissue microenvironment of human pancreatic cancer. Cancer Res 75:2629–2640. https://doi.org/10.1158/0008-5472.CAN-14-2921
Dong H, Strome SE, Salomao DR et al (2002) Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800. https://doi.org/10.1038/nm730
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174. https://doi.org/10.1038/nri2506
Ady JW, Heffner J, Klein E, Fong Y (2014) Oncolytic viral therapy for pancreatic cancer: current research and future directions. Oncolytic Virother 3:35–46. https://doi.org/10.2147/OV.S53858
Nattress CB, Hallden G (2018) Advances in oncolytic adenovirus therapy for pancreatic cancer. Cancer Lett 434:56–69. https://doi.org/10.1016/j.canlet.2018.07.006
Haller SD, Monaco ML, Essani K (2020) The present status of immuno-oncolytic viruses in the treatment of pancreatic cancer. Viruses 12:1318. https://doi.org/10.3390/v12111318
Kawashima T, Kagawa S, Kobayashi N et al (2004) Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res 10:285–292. https://doi.org/10.1158/1078-0432.ccr-1075-3
Hashimoto Y, Watanabe Y, Shirakiya Y et al (2008) Establishment of biological and pharmacokinetic assays of telomerase-specific replication-selective adenovirus. Cancer Sci 99:385–390. https://doi.org/10.1111/j.1349-7006.2007.00665.x
Yamasaki Y, Tazawa H, Hashimoto Y et al (2012) A novel apoptotic mechanism of genetically engineered adenovirus-mediated tumour-specific p53 overexpression through E1A-dependent p21 and MDM2 suppression. Eur J Cancer 48:2282–2291. https://doi.org/10.1016/j.ejca.2011.12.020
Hasei J, Sasaki T, Tazawa H et al (2013) Dual programmed cell death pathways induced by p53 transactivation overcome resistance to oncolytic adenovirus in human osteosarcoma cells. Mol Cancer Ther 12:314–325. https://doi.org/10.1158/1535-7163.MCT-12-0869
Koujima T, Tazawa H, Ieda T et al (2020) Oncolytic virus-mediated targeting of the ERK signaling pathway inhibits invasive propensity in human pancreatic cancer. Mol Ther Oncolytics 17:107–117. https://doi.org/10.1016/j.omto.2020.03.016
Kanaya N, Kuroda S, Kakiuchi Y et al (2020) Immune modulation by telomerase-specific oncolytic adenovirus synergistically enhances antitumor efficacy with Anti-PD1 antibody. Mol Ther 28:794–804. https://doi.org/10.1016/j.ymthe.2020.01.003
Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH (2012) Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21:822–835. https://doi.org/10.1016/j.ccr.2012.04.025
Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D (2012) Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21:836–847. https://doi.org/10.1016/j.ccr.2012.04.024
Schreck R, Baeuerle PA (1990) NF-kappa B as inducible transcriptional activator of the granulocyte-macrophage colony-stimulating factor gene. Mol Cell Biol 10:1281–1286. https://doi.org/10.1128/mcb.10.3.1281-1286.1990
Nomi T, Sho M, Akahori T et al (2007) Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 13:2151–2157. https://doi.org/10.1158/1078-0432.CCR-06-2746
Danilova L, Ho WJ, Zhu Q et al (2019) Programmed cell death ligand-1 (PD-L1) and CD8 expression profiling identify an immunologic subtype of pancreatic ductal adenocarcinomas with favorable survival. Cancer Immunol Res 7:886–895. https://doi.org/10.1158/2326-6066.CIR-18-0822
Sato H, Jeggo PA, Shibata A (2019) Regulation of programmed death-ligand 1 expression in response to DNA damage in cancer cells: Implications for precision medicine. Cancer Sci 110:3415–3423. https://doi.org/10.1111/cas.14197
Wang F, Ma J, Liu J, Jin H, Huang D (2012) Synthetic small peptides acting on B7H1 enhance apoptosis in pancreatic cancer cells. Mol Med Rep 6:553–557. https://doi.org/10.3892/mmr.2012.970
Vonderheide RH, Bear AS (2020) Tumor-derived myeloid cell chemoattractants and T cell exclusion in pancreatic cancer. Front Immunol 11:605619. https://doi.org/10.3389/fimmu.2020.605619
Ghansah T, Vohra N, Kinney K, Weber A, Kodumudi K, Springett G, Sarnaik AA, Pilon-Thomas S (2013) Dendritic cell immunotherapy combined with gemcitabine chemotherapy enhances survival in a murine model of pancreatic carcinoma. Cancer Immunol Immunother 62:1083–1091. https://doi.org/10.1007/s00262-013-1407-9
Eriksson E, Wenthe J, Irenaeus S, Loskog A, Ullenhag G (2016) Gemcitabine reduces MDSCs, tregs and TGFbeta-1 while restoring the teff/treg ratio in patients with pancreatic cancer. J Transl Med 14:282. https://doi.org/10.1186/s12967-016-1037-z
Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P (2012) Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother 61:1373–1385. https://doi.org/10.1007/s00262-011-1178-0
Christmas BJ, Rafie CI, Hopkins AC et al (2018) Entinostat converts immune-resistant breast and pancreatic cancers into checkpoint-responsive tumors by reprogramming tumor-infiltrating MDSCs. Cancer Immunol Res 6:1561–1577. https://doi.org/10.1158/2326-6066.CIR-18-0070
Wu C, Tan X, Hu X, Zhou M, Yan J, Ding C (2020) Tumor microenvironment following gemcitabine treatment favors differentiation of immunosuppressive Ly6C(high) myeloid cells. J Immunol 204:212–223. https://doi.org/10.4049/jimmunol.1900930
Fultang N, Li X, Li T, Chen YH (2020) Myeloid-derived suppressor cell differentiation in cancer: transcriptional regulators and enhanceosome-mediated mechanisms. Front Immunol 11:619253. https://doi.org/10.3389/fimmu.2020.619253
Mace TA, Ameen Z, Collins A et al (2013) Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res 73:3007–3018. https://doi.org/10.1158/0008-5472.CAN-12-4601
Ogawa T, Kikuchi S, Tabuchi M et al (2022) Modulation of p53 expression in cancer-associated fibroblasts prevents peritoneal metastasis of gastric cancer. Mol Ther Oncolytics 25:249–261. https://doi.org/10.1016/j.omto.2022.04.009
Tan MC, Goedegebuure PS, Belt BA, Flaherty B, Sankpal N, Gillanders WE, Eberlein TJ, Hsieh CS, Linehan DC (2009) Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol 182:1746–1755. https://doi.org/10.4049/jimmunol.182.3.1746
Wang X, Lang M, Zhao T et al (2017) Cancer-FOXP3 directly activated CCL5 to recruit FOXP3(+)Treg cells in pancreatic ductal adenocarcinoma. Oncogene 36:3048–3058. https://doi.org/10.1038/onc.2016.458
Wang X, Li X, Wei X et al (2020) PD-L1 is a direct target of cancer-FOXP3 in pancreatic ductal adenocarcinoma (PDAC), and combined immunotherapy with antibodies against PD-L1 and CCL5 is effective in the treatment of PDAC. Signal Transduct Target Ther 5:38. https://doi.org/10.1038/s41392-020-0144-8
Bhattacharya P, Budnick I, Singh M, Thiruppathi M, Alharshawi K, Elshabrawy H, Holterman MJ, Prabhakar BS (2015) Dual role of GM-CSF as a pro-inflammatory and a regulatory cytokine: implications for immune therapy. J Interferon Cytokine Res 35:585–599. https://doi.org/10.1089/jir.2014.0149
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I (2004) High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res 64:6337–6343. https://doi.org/10.1158/0008-5472.CAN-04-0757
Tsujikawa T, Crocenzi T, Durham JN et al (2020) Evaluation of cyclophosphamide/gvax pancreas followed by Listeria–Mesothelin (CRS-207) with or without Nivolumab in patients with pancreatic cancer. Clin Cancer Res 26:3578–3588. https://doi.org/10.1158/1078-0432.CCR-19-3978
Jiang J, Zhou H, Ni C, Hu X, Mou Y, Huang D, Yang L (2019) Immunotherapy in pancreatic cancer: new hope or mission impossible? Cancer Lett 445:57–64. https://doi.org/10.1016/j.canlet.2018.10.045
Azad A, Yin Lim S, D’Costa Z, et al (2017) PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol Med 9:167–180. https://doi.org/10.15252/emmm.201606674
Sugiu K, Tazawa H, Hasei J et al (2021) Oncolytic virotherapy reverses chemoresistance in osteosarcoma by suppressing MDR1 expression. Cancer Chemother Pharmacol 88:513–524. https://doi.org/10.1007/s00280-021-04310-5
Liu D, Kojima T, Ouchi M, Kuroda S, Watanabe Y, Hashimoto Y, Onimatsu H, Urata Y, Fujiwara T (2009) Preclinical evaluation of synergistic effect of telomerase-specific oncolytic virotherapy and gemcitabine for human lung cancer. Mol Cancer Ther 8:980–987. https://doi.org/10.1158/1535-7163.MCT-08-0901
Acknowledgements
We thank Tomoko Sueishi, Yuko Hoshijima, and Tae Yamanishi for their excellent technical support.
Funding
This study was supported in part by grants from the Japan Agency for Medical Research and Development (17ck0106285h0001 and 20ck0106569h0001 to T. Fujiwara) and JSPS KAKENHI grants JP16K10596 and JP21K07219 to H. Tazawa and JP16H05416 and JP19H03731 to T. Fujiwara.
Author information
Authors and Affiliations
Contributions
Conception and design: HT, SKa, TFuj; development of methodology: YK, MY, NK, TFus; acquisition of data: YK, MY, TFus; analysis and interpretation of data: YK, MY, TFus; writing, review, and/or revision of the manuscript: YK, HT, TFuj; administrative, technical, or material support: YU; study supervision: HT, SKi, SKu, TO, KN, RY, YU, SKa, TFuj
Corresponding author
Ethics declarations
Competing interests
Y. Urata is President & CEO of Oncolys BioPharma Inc. H. Tazawa and T. Fujiwara are consultants of Oncolys BioPharma Inc. The other authors have no potential conflicts of interest to disclose.
Conflict of interest
Y. Urata is President & CEO of Oncolys BioPharma Inc. H. Tazawa and T. Fujiwara are consultants of Oncolys BioPharma Inc. The other authors have no potential conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kajiwara, Y., Tazawa, H., Yamada, M. et al. Oncolytic virus-mediated reducing of myeloid-derived suppressor cells enhances the efficacy of PD-L1 blockade in gemcitabine-resistant pancreatic cancer. Cancer Immunol Immunother 72, 1285–1300 (2023). https://doi.org/10.1007/s00262-022-03334-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03334-x