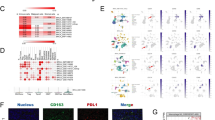
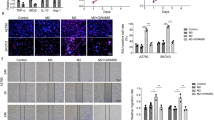
Abstract
Evidence has been presented demonstrating that CD8+ T cells confer anti-cancer effects, which offers a promising approach to enhance immunotherapy. M2-polarized tumor-associated macrophages (TAMs) could transfer RNA to cancer cells by secreting extracellular vesicles (EVs) and stimulate immune escape of cancer cells. Thus, the current study aimed at exploring how EVs derived from M2-polarized TAMs (M2-TAMs) affected the proliferation of ovarian cancer (OC) cells and apoptosis of CD8+ T cells. M2-TAMs were observed in OC tissues, which promoted proliferation of OC cells and CD8+ T cell apoptosis by secreting EVs. OC-associated differentially expressed gene NEAT1 was screened by bioinformatics analysis. The in vitro and in vivo effects of TAM-EVs-NEAT1 and its regulatory mechanism were assessed using gain- and loss-of-function assays in co-culture systems of TAMs-derived EVs, OC cells, and CD8+ T cells and in tumor-bearing mice. NEAT1 was highly expressed in M2-derived EVs and OC cells co-cultured with M2-derived EVs. NEAT1 sponged miR-101-3p to increase ZEB1 and PD-L1 expression. In vitro and in vivo assays confirmed the tumor-supporting effects of NEAT1 delivered by M2-derived EVs on OC cell proliferation and CD8+ T cell apoptosis as well as tumor growth. Collectively, M2-derived EVs containing NEAT1 exerted a tumor-promoting role in OC via the miR-101-3p/ZEB1/PD-L1 axis.








Similar content being viewed by others
Data availability
The data and materials of the study can be obtained from the corresponding author upon request.
References
Webb PM, Jordan SJ (2017) Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 41:3–14. https://doi.org/10.1016/j.bpobgyn.2016.08.006
Yigit R, Massuger LF, Figdor CG, Torensma R (2010) Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol 117:366–372. https://doi.org/10.1016/j.ygyno.2010.01.019
Farhood B, Najafi M, Mortezaee K (2019) CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol 234:8509–8521. https://doi.org/10.1002/jcp.27782
Barber E, Matei D (2021) Immunotherapy in ovarian cancer: we are not there yet. Lancet Oncol 22:903–905. https://doi.org/10.1016/S1470-2045(21)00303-X
Shu Y, Cheng P (2020) Targeting tumor-associated macrophages for cancer immunotherapy. Biochim Biophys Acta Rev Cancer 1874:188434. https://doi.org/10.1016/j.bbcan.2020.188434
Nowak M, Klink M (2020) The role of tumor-associated macrophages in the progression and chemoresistance of ovarian cancer. Cells. https://doi.org/10.3390/cells9051299
Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, Wang H, Wang K, Lin Y, Wang X (2018) Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res 6:1578–1592. https://doi.org/10.1158/2326-6066.CIR-17-0479
Cocozza F, Grisard E, Martin-Jaular L, Mathieu M, Thery C (2020) SnapShot: extracellular vesicles. Cell 182(262–262):e261. https://doi.org/10.1016/j.cell.2020.04.054
Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ (2018) Extracellular vesicles in cancer–implications for future improvements in cancer care. Nat Rev Clin Oncol 15:617–638. https://doi.org/10.1038/s41571-018-0036-9
Ghafouri-Fard S, Taheri M (2019) Nuclear enriched abundant transcript 1 (NEAT1): a long non-coding RNA with diverse functions in tumorigenesis. Biomed Pharmacother 111:51–59. https://doi.org/10.1016/j.biopha.2018.12.070
Chen X, Zhang S, Du K et al (2021) Gastric cancer-secreted exosomal X26nt increases angiogenesis and vascular permeability by targeting VE-cadherin. Cancer Sci 112:1839–1852. https://doi.org/10.1111/cas.14740
Yin L, Wang Y (2021) Long non-coding RNA NEAT1 facilitates the growth, migration, and invasion of ovarian cancer cells via the let-7 g/MEST/ATGL axis. Cancer Cell Int 21:437. https://doi.org/10.1186/s12935-021-02018-3
Zhao Y, Yu Z, Ma R et al (2021) lncRNA-Xist/miR-101-3p/KLF6/C/EBPalpha axis promotes TAM polarization to regulate cancer cell proliferation and migration. Mol Ther Nucleic Acids 23:536–551. https://doi.org/10.1016/j.omtn.2020.12.005
Zhao E, Maj T, Kryczek I et al (2016) Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol 17:95–103. https://doi.org/10.1038/ni.3313
Liang Y, Liu Y, Zhang Q, Zhang H, Du J (2021) Tumor-derived extracellular vesicles containing microRNA-1290 promote immune escape of cancer cells through the Grhl2/ZEB1/PD-L1 axis in gastric cancer. Transl Res 231:102–112. https://doi.org/10.1016/j.trsl.2020.12.003
Li CW, Lim SO, Xia W et al (2016) Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun 7:12632. https://doi.org/10.1038/ncomms12632
Shang A, Wang W, Gu C et al (2019) Long non-coding RNA HOTTIP enhances IL-6 expression to potentiate immune escape of ovarian cancer cells by upregulating the expression of PD-L1 in neutrophils. J Exp Clin Cancer Res 38:411. https://doi.org/10.1186/s13046-019-1394-6
Xue C, Xu Y, Ye W, Xie Q, Gao H, Xu B, Zhang D, Jiang J (2020) Expression of PD-L1 in ovarian cancer and its synergistic antitumor effect with PARP inhibitor. Gynecol Oncol 157:222–233. https://doi.org/10.1016/j.ygyno.2019.12.012
Qu QX, Xie F, Huang Q, Zhang XG (2017) Membranous and cytoplasmic expression of PD-L1 in ovarian cancer cells. Cell Physiol Biochem 43:1893–1906. https://doi.org/10.1159/000484109
Lu J, Liu QH, Wang F, Tan JJ, Deng YQ, Peng XH, Liu X, Zhang B, Xu X, Li XP (2018) Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J Exp Clin Cancer Res 37:147. https://doi.org/10.1186/s13046-018-0814-3
Zhao L, Liu Y, Zhang J, Liu Y, Qi Q (2019) LncRNA SNHG14/miR-5590-3p/ZEB1 positive feedback loop promoted diffuse large B cell lymphoma progression and immune evasion through regulating PD-1/PD-L1 checkpoint. Cell Death Dis 10:731. https://doi.org/10.1038/s41419-019-1886-5
Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, Epstein AL (2011) Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med 9:90. https://doi.org/10.1186/1479-5876-9-90
Lesokhin AM, Hohl TM, Kitano S et al (2012) Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res 72:876–886. https://doi.org/10.1158/0008-5472.CAN-11-1792
Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, Feng F, Liu Y, Xu W, Li Y (2019) Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res 38:81. https://doi.org/10.1186/s13046-019-1095-1
Li X, Tang M (2020) Exosomes released from M2 macrophages transfer miR-221-3p contributed to EOC progression through targeting CDKN1B. Cancer Med 9:5976–5988. https://doi.org/10.1002/cam4.3252
Liu Y, Wang Y, Fu X, Lu Z (2018) Long non-coding RNA NEAT1 promoted ovarian cancer cells’ metastasis through regulation of miR-382-3p/ROCK1 axial. Cancer Sci 109:2188–2198. https://doi.org/10.1111/cas.13647
Zhou D, Gu J, Wang Y, Wu H, Cheng W, Wang Q, Zheng G, Wang X (2021) Long non-coding RNA NEAT1 transported by extracellular vesicles contributes to breast cancer development by sponging microRNA-141-3p and regulating KLF12. Cell Biosci 11:68. https://doi.org/10.1186/s13578-021-00556-x
Xu H, Sun X, Huang Y, Si Q, Li M (2020) Long noncoding RNA NEAT1 modifies cell proliferation, colony formation, apoptosis, migration and invasion via the miR4500/BZW1 axis in ovarian cancer. Mol Med Rep 22:3347–3357. https://doi.org/10.3892/mmr.2020.11408
An J, Lv W, Zhang Y (2017) LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther 10:5377–5390. https://doi.org/10.2147/OTT.S147586
Xu Y, Xu L, Zheng J, Geng L, Zhao S (2017) MiR-101 inhibits ovarian carcinogenesis by repressing the expression of brain-derived neurotrophic factor. FEBS Open Bio 7:1258–1266. https://doi.org/10.1002/2211-5463.12257
Liang H, Yu T, Han Y et al (2018) LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol Cancer 17:119. https://doi.org/10.1186/s12943-018-0870-5
Cui Y, Qin L, Tian D, Wang T, Fan L, Zhang P, Wang Z (2018) ZEB1 promotes chemoresistance to cisplatin in ovarian cancer cells by suppressing SLC3A2. Chemotherapy 63:262–271. https://doi.org/10.1159/000493864
Zhan FL, Chen CF, Yao MZ (2020) LncRNA TUG1 facilitates proliferation, invasion and stemness of ovarian cancer cell via miR-186-5p/ZEB1 axis. Cell Biochem Funct 38:1069–1078. https://doi.org/10.1002/cbf.3544
Cortes M, Sanchez-Moral L, de Barrios O et al (2017) Tumor-associated macrophages (TAMs) depend on ZEB1 for their cancer-promoting roles. EMBO J 36:3336–3355. https://doi.org/10.15252/embj.201797345
Lv H, Lv G, Chen C et al (2021) NAD(+) metabolism maintains inducible PD-L1 expression to drive tumor immune evasion. Cell Metab 33(110–127):e115. https://doi.org/10.1016/j.cmet.2020.10.021
Zhang H, Qin G, Zhang C et al (2021) TRAIL promotes epithelial-to-mesenchymal transition by inducing PD-L1 expression in esophageal squamous cell carcinomas. J Exp Clin Cancer Res 40:209. https://doi.org/10.1186/s13046-021-01972-0
Lu L, Ling W, Ruan Z (2021) TAM-derived extracellular vesicles containing microRNA-29a-3p explain the deterioration of ovarian cancer. Mol Ther Nucleic Acids 25:468–482. https://doi.org/10.1016/j.omtn.2021.05.011
Funding
None.
Author information
Authors and Affiliations
Contributions
YW and LY contributed to the conception and design of the study; YW and LY contributed to the acquisition of data; YW and LY contributed to the analysis and interpretation of data; YW and LY contributed to drafting the article; YW and LY contributed to revising the article critically for important intellectual content; All of the authors approved the final version to be submitted.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, L., Wang, Y. Extracellular vesicles derived from M2-polarized tumor-associated macrophages promote immune escape in ovarian cancer through NEAT1/miR-101-3p/ZEB1/PD-L1 axis. Cancer Immunol Immunother 72, 743–758 (2023). https://doi.org/10.1007/s00262-022-03305-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03305-2