Abstract
Background
Few real-world data are available in patients with advanced metastatic non-small cell lung cancer (NSCLC) treated with first-line immunotherapy, particularly in those with brain metastases at treatment initiation.
Methods
This was a national, retrospective, multicenter study that consecutively included all patients with PD-L1-positive (tumor proportion score ≥ 50%) advanced NSCLC who initiated first-line treatment with pembrolizumab as a single agent between May 2017 (date of availability of pembrolizumab in this indication in France) to November 22, 2019 (approval of the pembrolizumab-chemotherapy combination). Data were collected from medical records with local response assessment.
Results
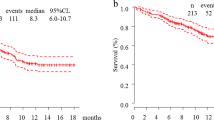
The cohort included 845 patients and 176 (20.8%) had brain metastases at diagnosis. There were no significant differences in outcomes for patients with and without brain metastases: 9.2 (95% CI 5.6–15) and 8 (95% CI 6.7–9.2, p = 0.3) months for median progression-free survival (PFS) and, 29.5 (95% CI 17.2–NA) and 22 (95% CI 17.8–27.1, p = 0.3) months for median overall survival (OS), respectively. Overall response rates were 47% and 45% in patients with and without cerebral metastases. In multivariate analysis, performance status 2–4 vs. 0–1 and neutrophil-to-lymphocyte ratio ≥ 4 vs. < 4 were the main independent negative factors for OS; brain metastasis was not an independent factor for OS.
Conclusion
In this large multicenter cohort, nearly 20% of patients initiating pembrolizumab therapy for advanced NSCLC had cerebral metastases. There was no significant difference in response rates, PFS and OS between patients with and without brain metastases.


Similar content being viewed by others
Availability of data and material
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Institut National du Cancer (2016) Accès aux tests moléculaires EGFR, RAS et BRAF. Résultats d’une enquête dans 5 régions françaises
Reck M, Rodriguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMoa1606774
Garon EB, Rizvi NA, Hui R et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028. https://doi.org/10.1056/NEJMoa1501824
Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL (2016) Pembrolizumab (Keytruda). Hum Vaccin Immunother 12:2777–2789. https://doi.org/10.1080/21645515.2016.1199310
Chatterjee M, Turner DC, Felip E et al (2016) Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 27:1291–1298. https://doi.org/10.1093/annonc/mdw174
Herbst RS, Baas P, Kim DW et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Pasello G, Pavan A, Attili I, Bortolami A, Bonanno L, Menis J, Conte P, Guarneri V (2020) Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat Rev 87:102031. https://doi.org/10.1016/j.ctrv.2020.102031
Reck M, Rodriguez-Abreu D, Robinson AG et al (2021) Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score >/= 50. J Clin Oncol 39:2339–2349. https://doi.org/10.1200/JCO.21.00174
Cortellini A, Tiseo M, Banna GL et al (2020) Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of >/= 50. Cancer Immunol Immunother 69:2209–2221. https://doi.org/10.1007/s00262-020-02613-9
Gounant V, Duruisseaux M, Soussi G et al (2021) Does very poor performance status systematically preclude single agent anti-PD-1 immunotherapy? A multicenter study of 35 consecutive patients. Cancers 13. https://doi.org/10.3390/cancers13051040
Roborel de Climens F, Chouaid C, Poulet C, Leroy V, Stoven L, Cortot AB, Dhalluin X, Gauvain C (2021) Salvage immunotherapy with pembrolizumab in patients hospitalized for life-threatening complications of NSCLC. JTO Clin Res Rep 2:100147. https://doi.org/10.1016/j.jtocrr.2021.100147
Wang B, Guo H, Xu H, Yu H, Chen Y, Zhao G (2021) Research progress and challenges in the treatment of central nervous system metastasis of non-small cell lung cancer. Cells 10. https://doi.org/10.3390/cells10102620
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265. https://doi.org/10.1016/S0140-6736(16)32517-X
Hellmann MD, Ciuleanu TE, Pluzanski A et al (2018) Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378:2093–2104. https://doi.org/10.1056/NEJMoa1801946
Carbone DP, Reck M, Paz-Ares L et al (2017) First-Line Nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 376:2415–2426. https://doi.org/10.1056/NEJMoa1613493
Langer CJ, Gadgeel SM, Borghaei H et al (2016) Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17:1497–1508. https://doi.org/10.1016/S1470-2045(16)30498-3
Govindan R, Szczesna A, Ahn MJ et al (2017) Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol 35:3449–3457. https://doi.org/10.1200/JCO.2016.71.7629
Gauvain C, Vauleon E, Chouaid C, Le Rhun E, Jabot L, Scherpereel A, Vinas F, Cortot AB, Monnet I (2018) Intracerebral efficacy and tolerance of nivolumab in non-small-cell lung cancer patients with brain metastases. Lung Cancer 116:62–66. https://doi.org/10.1016/j.lungcan.2017.12.008
Loiola T, de Alencar V, Guedes Camandaroba MP, Pirolli R, Fogassa CAZ, Cordeiro de Lima VC (2021) Immunotherapy as single treatment for patients with NSCLC With brain metastases: a systematic review and meta-analysis-the META-L-BRAIN study. J Thorac Oncol 16:1379–1391. https://doi.org/10.1016/j.jtho.2021.04.014
Goldberg SB, Gettinger SN, Mahajan A et al (2016) Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 17:976–983. https://doi.org/10.1016/S1470-2045(16)30053-5
Mansfield AS, Herbst RS, de Castro G Jr et al (2021) Outcomes with pembrolizumab monotherapy in patients with programmed death-ligand 1-positive NSCLC with brain metastases: pooled analysis of KEYNOTE-001, 010, 024, and 042. JTO Clin Res Rep 2: 100205. https://doi.org/10.1016/j.jtocrr.2021.100205
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RD, LG, CC and CD. The first draft of the manuscript was written by RD, CC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
L. Greillier reports grants, personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, Roche, Sanofi Aventis, Bristol-Myers Squibb, Merck Sharp & Dohme, Lilly, Novartis, Pfizer, Takeda, Bayer and Amgen, outside the submitted work. C. Chouaid reports grants, personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, GSK, Roche, Sanofi Aventis, Bristol-Myers Squibb, Merck Sharp & Dohme, Lilly, Novartis, Pfizer, Takeda, Bayer and Amgen, outside the submitted work. C. Decroisette reports personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, Roche, Sanofi Aventis, Bristol-Myers Squibb, Merck Sharp & Dohme, Lilly, Novartis, Pfizer, Takeda, and Amgen, outside the submitted work. M. Pérol reports personal fees and non-financial support from Roche, Eli Lilly, Pfizer, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Novartis, AstraZeneca, Takeda, Gritstone, Sanofi, GlaxoSmithKline, Amgen, Chugai, Illumina, Daïchi-Sankyo and Abbvie outside the submitted work. R. Descourt reports personal fees and non-financial support from AstraZeneca, Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, Takeda, and Chugai, outside the submitted work. C. Ricordel, J.B. Auliac, L. Falchero, R. Gervais, R. Veillon, S. Vieillot, F. Guisier, M. Marcq, G. Justeau, L. Bigay-Game, M. Bernardi, P. Fournel, H. Doubre, J. Pinsolle and K. Amrane report no conflict of interest.
Ethical approval
The study conformed to the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. It was approved by a national independent Ethics Committee (2019-A02073-54, on December, 11, 2019).
Consent to participate
Patients received written and oral information on the study and gave their consent to participate in the study and for the use of their medical data for research purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Descourt, R., Greillier, L., Perol, M. et al. First-line single-agent pembrolizumab for PD-L1-positive (tumor proportion score ≥ 50%) advanced non-small cell lung cancer in the real world: impact in brain metastasis: a national French multicentric cohort (ESCKEYP GFPC study). Cancer Immunol Immunother 72, 91–99 (2023). https://doi.org/10.1007/s00262-022-03232-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03232-2