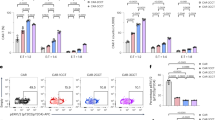
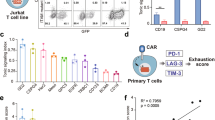
Abstract
Chimeric antigen receptor (CAR) T-cell therapy achieves great success for hematological malignancies. However, clinical trials have revealed some limitations in both improving the efficacy and reducing the relapse, which calls for innovative strategies to engineer more powerful CAR-T cells. Promoting the formation of CAR clusters provides an alternative approach and potentially improves current CAR T-cell therapy against cancers. Here, we generated CARCys-T cells using a 4-1BB-derived hinge region including 11 cysteines residues. The cysteines in the hinge were found to facilitate CARCys clustering upon antigen stimulation and promote the antitumor activity of CAR-T cells. Compared with most conventionally used CAR-T cells with CD8α-derived hinge (CARconv-T cells), CARCys-T cells exhibited larger diameter of CAR clusters and enhanced antigen-specific tumor lysis both in vitro and in vivo. In addition, the CARCys-mediated enhancement could be applied to HER2, CD19 as well as GPC3-targeted CAR-T cells. More importantly, CARCys-T cells showed potent antitumor efficacy in clinically relevant patient-derived primary tumor cells and organoids. Thus, the novel hinge containing 11 cysteines provides a promising strategy to facilitate CAR clustering and maximize anti-tumor activity of CAR-T cells, which emphasizes the importance of CAR clustering to improve CAR T-cell therapy in the clinic.





Similar content being viewed by others
References
June CH, Sadelain M (2018) Chimeric antigen receptor therapy. N Engl J Med 379:64–73. https://doi.org/10.1056/NEJMra1706169
Jackson HJ, Rafiq S, Brentjens RJ (2016) Driving CAR T-cells forward. Nat Rev Clin Oncol 13:370–383. https://doi.org/10.1038/nrclinonc.2016.36
Majzner RG, Mackall CL (2019) Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med 25:1341–1355. https://doi.org/10.1038/s41591-019-0564-6
Hong M, Clubb JD, Chen YY (2020) Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell 38:473–488. https://doi.org/10.1016/j.ccell.2020.07.005
Sadelain M, Brentjens R, Riviere I (2013) The basic principles of chimeric antigen receptor design. Cancer Discov 3:388–398. https://doi.org/10.1158/2159-8290.CD-12-0548
Moritz D, Wels W, Mattern J, Groner B (1994) Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells. Proc Natl Acad Sci USA 91:4318–4322. https://doi.org/10.1073/pnas.91.10.4318
Arakawa F, Shibaguchi H, Xu Z, Kuroki M (2002) Targeting of T cells to CEA-expressing tumor cells by chimeric immune receptors with a highly specific single-chain anti-CEA activity. Anticancer Res 22:4285–4289
Eshhar Z, Waks T, Gross G, Schindler DG (1993) Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA 90:720–724. https://doi.org/10.1073/pnas.90.2.720
Ramos CA, Heslop HE, Brenner MK (2016) CAR-T cell therapy for lymphoma. Annu Rev Med 67:165–183. https://doi.org/10.1146/annurev-med-051914-021702
Srivastava S, Riddell SR (2015) Engineering CAR-T cells: design concepts. Trends Immunol 36:494–502. https://doi.org/10.1016/j.it.2015.06.004
Davenport AJ, Cross RS, Watson KA et al (2018) Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc Natl Acad Sci USA 115:E2068–E2076. https://doi.org/10.1073/pnas.1716266115
Li R, Ma C, Cai H, Chen W (2020) The CAR T-Cell mechanoimmunology at a Glance. Adv Sci 7:2002628. https://doi.org/10.1002/advs.202002628
Wu L, Wei Q, Brzostek J, Gascoigne NRJ (2020) Signaling from T cell receptors (TCRs) and chimeric antigen receptors (CARs) on T cells. Cell Mol Immunol 17:600–612. https://doi.org/10.1038/s41423-020-0470-3
Gudipati V, Rydzek J, Doel-Perez I et al (2020) Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat Immunol 21:848–856. https://doi.org/10.1038/s41590-020-0719-0
Guedan S, Chen X, Madar A et al (2014) ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood 124:1070–1080. https://doi.org/10.1182/blood-2013-10-535245
Johnson LA, Scholler J, Ohkuri T et al (2015) Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaa4963
Long AH, Haso WM, Shern JF et al (2015) 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 21:581–590. https://doi.org/10.1038/nm.3838
Maher J, Wilkie S, Davies DM, Arif S, Picco G, Julien S, Foster J, Burchell J, Taylor-Papadimitriou J (2016) Targeting of tumor-associated glycoforms of MUC1 with CAR T cells. Immunity 45:945–946. https://doi.org/10.1016/j.immuni.2016.10.014
Goyette J, Nieves DJ, Ma Y, Gaus K (2019) How does T cell receptor clustering impact on signal transduction? J Cell Sci. https://doi.org/10.1242/jcs.226423
Ma Y, Lim YJ, Benda A, Lou J, Goyette J, Gaus K (2020) Clustering of the zeta-Chain can initiate T cell receptor signaling. Int J Mol Sci. https://doi.org/10.3390/ijms21103498
Ma Y, Pandzic E, Nicovich PR, Yamamoto Y, Kwiatek J, Pageon SV, Benda A, Rossy J, Gaus K (2017) An intermolecular FRET sensor detects the dynamics of T cell receptor clustering. Nat Commun 8:15100. https://doi.org/10.1038/ncomms15100
Fass D, Thorpe C (2018) Chemistry and enzymology of disulfide cross-linking in proteins. Chem Rev 118:1169–1198. https://doi.org/10.1021/acs.chemrev.7b00123
Hogg PJ (2020) Multiple disulfide-bonded states of native proteins: estimate of number using probabilities of disulfide bond formation. Molecules. https://doi.org/10.3390/molecules25235729
Guest RD, Hawkins RE, Kirillova N et al (2005) The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother 28:203–211. https://doi.org/10.1097/01.cji.0000161397.96582.59
Zhang X, Calvert RA, Sutton BJ, Dore KA (2017) IgY: a key isotype in antibody evolution. Biol Rev Camb Philos Soc 92:2144–2156. https://doi.org/10.1111/brv.12325
Bitra A, Doukov T, Wang J, Picarda G, Benedict CA, Croft M, Zajonc DM (2018) Crystal structure of murine 4–1BB and its interaction with 4–1BBL support a role for galectin-9 in 4–1BB signaling. J Biol Chem 293:1317–1329. https://doi.org/10.1074/jbc.M117.814905
Luo F, Qian J, Yang J, Deng Y, Zheng X, Liu J, Chu Y (2016) Bifunctional alphaHER2/CD3 RNA-engineered CART-like human T cells specifically eliminate HER2(+) gastric cancer. Cell Res 26:850–853. https://doi.org/10.1038/cr.2016.81
Ester M, Kriegel HP, Sander J, Xu X (1996) A Density-Based Algorithm for Discovering Clusters in Large Spatial Databases with Noise. AAAI Press. In: Proceedings of the 2nd International Conference on Knowledge Discovery and Data Mining, pp 226–231
Broutier L, Mastrogiovanni G, Verstegen MM et al (2017) Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med 23:1424–1435. https://doi.org/10.1038/nm.4438
Cattaneo CM, Dijkstra KK, Fanchi LF, Kelderman S, Kaing S, van Rooij N, van den Brink S, Schumacher TN, Voest EE (2020) Tumor organoid-T-cell coculture systems. Nat Protoc 15:15–39. https://doi.org/10.1038/s41596-019-0232-9
Wang LC, Lo A, Scholler J et al (2014) Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res 2:154–166. https://doi.org/10.1158/2326-6066.CIR-13-0027
Qin L, Lai Y, Zhao R et al (2017) Incorporation of a hinge domain improves the expansion of chimeric antigen receptor T cells. J Hematol Oncol 10:68. https://doi.org/10.1186/s13045-017-0437-8
Majzner RG, Rietberg SP, Sotillo E et al (2020) Tuning the antigen density requirement for CAR T-cell activity. Cancer Discov 10:702–723. https://doi.org/10.1158/2159-8290.CD-19-0945
Brudno JN, Kochenderfer JN (2018) Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 15:31–46. https://doi.org/10.1038/nrclinonc.2017.128
Saito T, Yokosuka T (2006) Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol 18:305–313. https://doi.org/10.1016/j.coi.2006.03.014
Gao Y, Wang Y, Luo F, Chu Y (2021) Optimization of T Cell redirecting strategies: obtaining inspirations from natural process of T cell activation. Front Immunol 12:664329. https://doi.org/10.3389/fimmu.2021.664329
Pageon SV, Tabarin T, Yamamoto Y et al (2016) Functional role of T-cell receptor nanoclusters in signal initiation and antigen discrimination. Proc Natl Acad Sci USA 113:E5454–E5463. https://doi.org/10.1073/pnas.1607436113
Taylor MJ, Husain K, Gartner ZJ, Mayor S, Vale RD (2017) A DNA-based T cell receptor reveals a role for receptor clustering in ligand discrimination. Cell 169:108–119. https://doi.org/10.1016/j.cell.2017.03.006
Singh N, Frey NV, Engels B et al (2021) Antigen-independent activation enhances the efficacy of 4–1BB-costimulated CD22 CAR T cells. Nat Med 27:842–850. https://doi.org/10.1038/s41591-021-01326-5
Alabanza L, Pegues M, Geldres C, Shi V, Wiltzius JJW, Sievers SA, Yang S, Kochenderfer JN (2017) Function of novel Anti-CD19 chimeric antigen receptors with human variable regions is affected by Hinge and transmembrane domains. Mol Ther J American Soc Gene Ther 25:2452–2465. https://doi.org/10.1016/j.ymthe.2017.07.013
Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR (2015) The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res 3:125–135. https://doi.org/10.1158/2326-6066.CIR-14-0127
Jonnalagadda M, Mardiros A, Urak R et al (2015) Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther J American Soc Gene Ther 23:757–768. https://doi.org/10.1038/mt.2014.208
Watanabe N, Bajgain P, Sukumaran S, Ansari S, Heslop HE, Rooney CM, Brenner MK, Leen AM, Vera JF (2016) Fine-tuning the CAR spacer improves T-cell potency. Oncoimmunology 5:e1253656. https://doi.org/10.1080/2162402X.2016.1253656
Acknowledgements
The authors thank National Center for Protein Science Shanghai (NCPSS) for STORM system, Prof. Yanhui Xu (Fudan University), Prof. Chenqi Xu (University of Chinese Academy of Sciences) and Ms. Yang Yu (NCPSS) for valuable suggestions, Dr. Yufei Zhang of Olympus (Beijing) sales & service co., Ms. Yao Li of NCPSS, Ms. Shenglan Gu of International Peace Maternity and Child Health Hospital for excellent technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China (82121002, 82130050, 81870375), Shanghai Rising-Star Program (18QA1401000), Open Research Fund of State Key Laboratory of Genetic Engineering, Fudan University (SKLGE1911), and Shenzhen Institute of Synthetic Biology Scientific Research Program (ZTXM20214002). SA is a Senior Clinical Investigator of the Research Fund-Flanders (FWO) (Belgium). GR is supported by a Doctoral Grant Strategic Basic Research of the FWO (Grant 1S72821N) and the MeToYou Foundation (Belgium).
Author information
Authors and Affiliations
Contributions
YC, FL, and YW designed the experiments. YW and FL wrote the manuscript. YC, FL, JL, SA, GR, and JQ revised the manuscript. YW, YG, CN, SZ, GR, LC, and CY performed the experiments and acquired the data. YW, FL, BW, JQ, and LC analyzed the data and prepared the figures. All authors provided intellectual input and read the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval and ethical standards.
Human N87, Huh7, Ramos and K562 cell lines were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Science (Shanghai, China). Human peripheral blood mononuclear cells isolated from healthy donors or patients with hepatocellular carcinoma (HCC) were purchased from the Shanghai Blood Center (Shanghai, China), or obtained from Huashan hospital (Shanghai, China), respectively. Fresh human HCC samples were obtained from surgery performed at the Huashan hospital. Informed consent was obtained from HCC patients involved, and the project was approved by the Ethical Committee of Medical Research, Huashan Hospital of Fudan University (No. 2013-005). Six- to eight-week-old female nude mice (BALB/c) were purchased from SLAC (Shanghai, China) and kept under specific pathogen-free conditions in animal facility of Fudan University. The animal experimental protocol was approved by the Ethics Committee of Fudan University (201901003Z) following the Guidelines for the Care and Use of Laboratory Animals (No. 55 issued by Ministry of Health, China on January 25th, 1998).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Gao, Y., Niu, C. et al. Chimeric antigen receptor clustering via cysteines enhances T-cell efficacy against tumor. Cancer Immunol Immunother 71, 2801–2814 (2022). https://doi.org/10.1007/s00262-022-03195-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03195-4