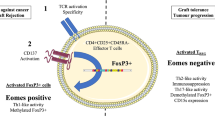
Abstract
Non-keratinizing nasopharyngeal carcinoma (NPC) is a malignancy with a poor prognosis for relapsing patients and those with metastatic disease. Here, we identify a novel disease mechanism of NPC which may be its Achilles’ heel that makes it susceptible to immunotherapy. CD137 is a potent costimulatory receptor on activated T cells, and CD137 agonists strongly enhance anti-tumor immune responses. A negative feedback mechanism prevents overstimulation by transferring CD137 from T cells to CD137 ligand (CD137L)-expressing antigen presenting cells (APC) during cognate interaction, upon which the CD137-CD137L complex is internalized and degraded. We found ectopic expression of CD137 on 42 of 122 (34.4%) NPC cases, and that CD137 is induced by the Epstein-Barr virus latent membrane protein (LMP) 1. CD137 expression enables NPC to hijack the inbuilt negative feedback mechanism to downregulate the costimulatory CD137L on APC, facilitating its escape from immune surveillance. Further, the ectopically expressed CD137 signals into NPC cells via the p38-MAPK pathway, and induces the expression of IL-6, IL-8 and Laminin γ2. As much as ectopic CD137 expression may support the growth and spread of NPC, it may be a target for its immunotherapeutic elimination. Natural killer cells that express a CD137-specific chimeric antigen receptor induce death in CD137+ NPC cells, in vitro, and in vivo in a murine xenograft model. These data identify a novel immune escape mechanism of NPC, and lay the foundation for an urgently needed immunotherapeutic approach for NPC.






Similar content being viewed by others
References
Smith C, Khanna R (2012) A new approach for cellular immunotherapy of nasopharyngeal carcinoma. Oncoimmunology 1(8):1440–1442
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Ma BB, Chan AT (2006) Systemic treatment strategies and therapeutic monitoring for advanced nasopharyngeal carcinoma. Expert Rev Anticancer Ther 6(3):383–394
Petersson F (2015) Nasopharyngeal carcinoma: a review. Semin Diagn Pathol 32(1):54–73
Lee AW, Ma BB, Ng WT, Chan AT (2015) Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol: Off J Am Soc Clin Oncol 33(29):3356–3364
Outh-Gauer S, Alt M, Le Tourneau C, Augustin J, Broudin C, Gasne C et al (2018) Immunotherapy in head and neck cancers: a new challenge for immunologists, pathologists and clinicians. Cancer Treat Rev 65:54–64
Ressing ME, Horst D, Griffin BD, Tellam J, Zuo J, Khanna R et al (2008) Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products. Semin Cancer Biol 18(6):397–408
Tagawa T, Albanese M, Bouvet M, Moosmann A, Mautner J, Heissmeyer V et al (2016) Epstein-Barr viral miRNAs inhibit antiviral CD4+ T cell responses targeting IL-12 and peptide processing. J Exp Med 213(10):2065–2080
Ho WT, Pang WL, Chong SM, Castella A, Al-Salam S, Tan TE et al (2013) Expression of CD137 on Hodgkin and Reed-Sternberg cells inhibits T-cell activation by eliminating CD137 ligand expression. Can Res 73(2):652–661
Thum E, Shao Z, Schwarz H (2009) CD137, implications in immunity and potential for therapy. Front Biosci 14:4173–4188
Lee SW, Croft M (2009) 4-1BB as a therapeutic target for human disease. In: Grewal IS (ed) Therapeutic targets of the TNF superfamily, vol 647. Springer, New York, pp 120–129
Wang S, Chen L (2011) Immunobiology of cancer therapies targeting CD137 and B7-H1/PD-1 cosignal pathways. Curr Top Microbiol Immunol 344:245–267
Sanmamed MF, Etxeberria I, Otano I, Melero I (2019) Twists and turns to translating 4-1BB cancer immunotherapy. Sci Transl Med. 11(496):eaax4738
Mbanwi AN, Watts TH (2014) Costimulatory TNFR family members in control of viral infection: outstanding questions. Sem Immunol 26(3):210–219
Lee S, Mittler RS, Moore ML (2014) Targeting CD137 enhances vaccine-elicited anti-respiratory syncytial virus CD8+ T cell responses in aged mice. J Immunol 192(1):293–299
Wang R, Freywald A, Chen Y, Xu J, Tan X, Xiang J (2015) Transgenic 4-1BBL-engineered vaccine stimulates potent Gag-specific therapeutic and long-term immunity via increased priming of CD44(+)CD62L(high) IL-7R(+) CTLs with up- and downregulation of anti- and pro-apoptosis genes. Cell Mol Immunol 12(4):456–465
Campana D, Schwarz H, Imai C (2014) 4-1BB chimeric antigen receptors. Cancer J 20(2):134–140
Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M et al (2015) 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 21(6):581–590
Yoshimori M, Imadome K, Komatsu H, Wang L, Saitoh Y, Yamaoka S et al (2014) CD137 expression is induced by Epstein-Barr virus infection through LMP1 in T or NK cells and mediates survival promoting signals. PLoS ONE 9(11):e112564
Wu M, Wong HY, Lin JL, Moliner A, Schwarz H (2019) Induction of CD137 expression by viral genes reduces T cell costimulation. J Cell Physiol 234(11):21076–21088
Shao Z, Harfuddin Z, Pang WL, Nickles E, Koh LK, Schwarz H (2015) Trogocytic CD137 transfer causes an internalization of CD137 ligand on murine APCs leading to reduced T cell costimulation. J Leukoc Biol 97(5):909–919
Luu K, Patwardhan MV, Zeng Q, Wickstrom SL, Lundqvist A, Schwarz H (2021) Regulatory T cells inhibit T cell activity by downregulating CD137 ligand via CD137 trogocytosis. Cells 10(2):353
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S et al (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N et al (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25(16):2078–2079
Liao Y, Smyth GK, Shi W (2013) The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res 41(10):e108
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):550
Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P (2015) The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1(6):417–425
Rajendran S, Li Y, Ngoh E, Wong HY, Cheng MS, Wang CI et al (2019) Development of a bispecific antibody targeting CD30 and CD137 on Hodgkin and Reed-Sternberg cells. Front Oncol 9:945
Broll K, Richter G, Pauly S, Hofstaedter F, Schwarz H (2001) CD137 expression in tumor vessel walls. High correlation with malignant tumors. Am J Clin Pathol 115(4):543–549
Aravinth SP, Rajendran S, Li Y, Wu M, Yi Wong AH, Schwarz H (2019) Epstein-Barr virus-encoded LMP1 induces ectopic CD137 expression on Hodgkin and Reed-Sternberg cells via the PI3K-AKT-mTOR pathway. Leuk Lymphoma 60(11):2697–2704
Rajendran S, Ho WT, Schwarz H (2016) CD137 signaling in Hodgkin and Reed-Sternberg cell lines induces IL-13 secretion, immune deviation and enhanced growth. Oncoimmunology 5(6):e1160188
Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M et al (2007) Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 110(1):201–210
Ye Q, Song DG, Powell DJ Jr (2013) Finding a needle in a haystack: Activation-induced CD137 expression accurately identifies naturally occurring tumor-reactive T cells in cancer patients. Oncoimmunology 2(12):e27184
Bacher P, Heinrich F, Stervbo U, Nienen M, Vahldieck M, Iwert C et al (2016) Regulatory T cell specificity directs tolerance versus allergy against aeroantigens in humans. Cell 167(4):1067–1078
Kachapati K, Adams DE, Wu Y, Steward CA, Rainbow DB, Wicker LS et al (2012) The B10 Idd9.3 locus mediates accumulation of functionally superior CD137(+) regulatory T cells in the nonobese diabetic type 1 diabetes model. J Immunol 189(10):5001–5015
Luu K, Nickles E, Schwarz H (2020) Destroy, what destroys you. Oncoimmunology 9(1):1685301
Saito K, Ohara N, Hotokezaka H, Fukumoto S, Yuasa K, Naito M et al (2004) Infection-induced up-regulation of the costimulatory molecule 4-1BB in osteoblastic cells and its inhibitory effect on M-CSF/RANKL-induced in vitro osteoclastogenesis. J Biol Chem 279(14):13555–13563
Pichler K, Kattan T, Gentzsch J, Kress AK, Taylor GP, Bangham CR et al (2008) Strong induction of 4-1BB, a growth and survival promoting costimulatory receptor, in HTLV-1-infected cultured and patients’ T cells by the viral Tax oncoprotein. Blood 111(9):4741–4751
Dharmadhikari B, Wu M, Abdullah NS, Rajendran S, Ishak ND, Nickles E et al (2016) CD137 and CD137L signals are main drivers of type 1, cell-mediated immune responses. Oncoimmunology. 5(4):e1113367
Lee KY, Wong HY, Zeng Q, Le Lin J, Cheng MS, Kuick CH et al (2021) Ectopic CD137 expression by rhabdomyosarcoma provides selection advantages but allows immunotherapeutic targeting. Oncoimmunology 10(1):1877459
Arch RH, Thompson CB (1998) 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol 18(1):558–565
Cannons JL, Choi Y, Watts TH (2000) Role of TNF receptor-associated factor 2 and p38 mitogen-activated protein kinase activation during 4-1BB-dependent immune response. J Immunol 165(11):6193–6204
Cannons JL, Hoeflich KP, Woodgett JR, Watts TH (1999) Role of the stress kinase pathway in signaling via the T cell costimulatory receptor 4-1BB. J Immunol 163(6):2990–2998
Chow KC, Chiou SH, Ho SP, Tsai MH, Chen CL, Wang LS et al (2003) The elevated serum interleukin-6 correlates with the increased serum butyrate level in patients with nasopharyngeal carcinoma. Oncol Rep 10(4):813–819
Zhang G, Tsang CM, Deng W, Yip YL, Lui VW, Wong SC et al (2013) Enhanced IL-6/IL-6R signaling promotes growth and malignant properties in EBV-infected premalignant and cancerous nasopharyngeal epithelial cells. PLoS ONE 8(5):e62284
Sun W, Liu DB, Li WW, Zhang LL, Long GX, Wang JF et al (2014) Interleukin-6 promotes the migration and invasion of nasopharyngeal carcinoma cell lines and upregulates the expression of MMP-2 and MMP-9. Int J Oncol 44(5):1551–1560
Li XJ, Peng LX, Shao JY, Lu WH, Zhang JX, Chen S et al (2012) As an independent unfavorable prognostic factor, IL-8 promotes metastasis of nasopharyngeal carcinoma through induction of epithelial–mesenchymal transition and activation of AKT signaling. Carcinogenesis 33(7):1302–1309
Yoshizaki T, Horikawa T, Qing-Chun R, Wakisaka N, Takeshita H, Sheen TS et al (2001) Induction of interleukin-8 by Epstein-Barr virus latent membrane protein-1 and its correlation to angiogenesis in nasopharyngeal carcinoma. Clin Cancer Res: Off J Am Assoc Cancer Res 7(7):1946–1951
Liao B, Zhong BL, Li Z, Tian XY, Li Y, Li B (2010) Macrophage migration inhibitory factor contributes angiogenesis by up-regulating IL-8 and correlates with poor prognosis of patients with primary nasopharyngeal carcinoma. J Surg Oncol 102(7):844–851
Xie LQ, Bian LJ, Li Z, Li Y, Liang YJ (2010) Co-elevated expression of hepatocyte growth factor and Interleukin-8 contributes to poor prognosis of patients with primary nasopharyngeal carcinoma. Oncol Rep 23(1):141–150
Horikawa T, Kaizaki Y, Kato H, Furukawa M, Yoshizaki T (2005) Expression of interleukin-8 receptor A predicts poor outcome in patients with nasopharyngeal carcinoma. Laryngoscope 115(1):62–67
Garg M, Braunstein G, Koeffler HP (2014) LAMC2 as a therapeutic target for cancers. Expert Opin Ther Targets 18(9):979–982
Moon YW, Rao G, Kim JJ, Shim HS, Park KS, An SS et al (2015) LAMC2 enhances the metastatic potential of lung adenocarcinoma. Cell Death Differ 22(8):1341–1352
Okada Y, Takahashi N, Takayama T, Goel A (2021) LAMC2 promotes cancer progression and gemcitabine resistance through modulation of EMT and ATP-binding cassette transporters in pancreatic ductal adenocarcinoma. Carcinogenesis 42(4):546–556
Buchan SL, Dou L, Remer M, Booth SG, Dunn SN, Lai C et al (2018) Antibodies to costimulatory receptor 4-1BB enhance anti-tumor immunity via T regulatory cell depletion and promotion of CD8 T cell effector function. Immunity 49(5):958–970
Freeman ZT, Nirschl TR, Hovelson DH, Johnston RJ, Engelhardt JJ, Selby MJ et al (2020) A conserved intratumoral regulatory T cell signature identifies 4-1BB as a pan-cancer target. J Clin Investig 130(3):1405–1416
Acknowledgements
This research was supported by a grant (NMRC/BnB/018b/2015) from the National Medical Research Council (NMRC), Singapore to H.S. and by A*STAR core funding and Singapore NRF under its NRF-SIS “SingMass” scheme to R.M.S. J.K.T. is supported by the Transition Award (TA20nov-0025) from NMRC, and K.S.L is supported by the Individual Research Grant (CIRG18nov-0045) from NMRC. We thank the LSI core facility under the leadership of Dr. Paul Hutchinson for excellent help with flow cytometry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prasad, M., Ponnalagu, S., Zeng, Q. et al. Epstein-Barr virus-induced ectopic CD137 expression helps nasopharyngeal carcinoma to escape immune surveillance and enables targeting by chimeric antigen receptors. Cancer Immunol Immunother 71, 2583–2596 (2022). https://doi.org/10.1007/s00262-022-03183-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03183-8