Abstract
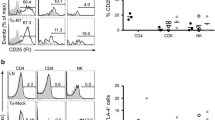
Talimogene Laherparepvec (OncoVEXmGMCSF), an oncolytic virus, immune checkpoint inhibitor anti-programmed cell death protein 1 (anti-PD1), and BRAF inhibition (BRAFi), are all clinically approved for treatment of melanoma patients and are effective through diverse mechanisms of action. Individually, these therapies also have an effect on the tumor immune microenvironment (TIME). Evaluating the combination effect of these three therapies on the TIME can help determine when combination therapy is most appropriate for further study. In this study, we use a transgenic murine melanoma model (Tyr::CreER; BRAFCA/+; PTENflox/flox), to evaluate the TIME in response to combinations of BRAFi, anti-PD1, and OncoVEXmGMCSF. We find that mice treated with the triple combination BRAFi + anti-PD1 + OncoVEXmGMCSF have decreased tumor growth compared to BRAFi alone and prolonged survival compared to control. Flow cytometry shows an increase in percent CD8 + /CD3 + cytotoxic T Lymphocytes (CTLs) and a decrease in percent FOXP3 + /CD4 + T regulatory cells (Tregs) in tumors treated with OncoVEXmGMCSF compared to mice not treated with OncoVEXmGMCSF. Immunogenomic analysis at 30d post-treatment shows an increase in Th1 and interferon-related genes in mice receiving OncoVEXmGMCSF + BRAFi. In summary, treatment with combination BRAFi + anti-PD1 + OncoVEXmGMCSF is more effective than any single treatment in controlling tumor growth, and groups receiving OncoVEXmGMCSF had more tumoral infiltration of CTLs and less intratumoral Tregs in the TIME. This study provides rational basis to combine targeted agents, oncolytic viral therapy, and checkpoint inhibitors in the treatment of melanoma.






Similar content being viewed by others
Data availability
All expression data is available in supplemental files. For any other data please email corresponding author.
Abbreviations
- 4-HT:
-
4-Hydroxytamoxifen
- anti-PD1:
-
Anti-programmed cell death protein 1
- ASBL-2:
-
Animal biosafety level 2
- BRAFi:
-
BRAF inhibition
- CTLs:
-
Cytotoxic T Lymphocytes
- CA:
-
Conditionally active
- CUIMC:
-
Columbia university irving medical center
- FFPE:
-
Formalin-fixed paraffin-embedded
- gC:
-
Glycoprotein C
- GM-CSF:
-
Human granulocyte–macrophage colony-stimulating factor
- HSV-1:
-
Herpes simplex virus type 1
- IACUC:
-
Institutional animal care and use committee
- IP:
-
Intraperitoneal injection
- IT:
-
Intratumoral injection
- PBS:
-
Phosphate buffered saline
- RT-PCR:
-
Transcription-polymerase chain reaction
- TIME:
-
Tumor immune microenvironment
- Tregs:
-
T regulatory cells
- OncoVEXmGMCSF :
-
Talimogene laherparepvec
References
Ascierto PA, Kirkwood JM, Grob JJ et al (2012) The role of BRAF V600 mutation in melanoma. J Transl Med 10:85. https://doi.org/10.1186/1479-5876-10-85
Dummer R, Ascierto PA, Gogas HJ et al (2018) Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 19:1315–1327. https://doi.org/10.1016/S1470-2045(18)30497-2
Ascierto PA, Ferrucci PF, Fisher R et al (2019) Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med 25:941–946. https://doi.org/10.1038/s41591-019-0448-9
Huynh S, Mortier L, Dutriaux C et al (2020) Combined Therapy with Anti-PD1 and BRAF and/or MEK Inhibitor for Advanced Melanoma: A Multicenter Cohort Study. Cancers (Basel) 12:1666. https://doi.org/10.3390/cancers12061666
Sun L, Funchain P, Song JM, Rayman P, Tannenbaum C, Ko J, McNamara M, Marcela Diaz-Montero C, Gastman B (2018) Talimogene Laherparepvec combined with anti-PD-1 based immunotherapy for unresectable stage III-IV melanoma: a case series. J Immunother Cancer 6:36. https://doi.org/10.1186/s40425-018-0337-7
Dummer R, Lebbe C, Atkinson V et al (2020) Combined PD-1, BRAF and MEK inhibition in advanced BRAF-mutant melanoma: safety run-in and biomarker cohorts of COMBI-i. Nat Med 26:1557–1563. https://doi.org/10.1038/s41591-020-1082-2
Bayan CY, Lopez AT, Gartrell RD et al (2018) The Role of Oncolytic Viruses in the Treatment of Melanoma. Curr Oncol Rep 20:80. https://doi.org/10.1007/s11912-018-0729-3
Bommareddy PK, Patel A, Hossain S, Kaufman HL (2017) Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am J Clin Dermatol 18:1–15. https://doi.org/10.1007/s40257-016-0238-9
Grigg C, Blake Z, Gartrell R, Sacher A, Taback B, Saenger Y (2016) Talimogene laherparepvec (T-Vec) for the treatment of melanoma and other cancers. Semin Oncol 43:638–646. https://doi.org/10.1053/j.seminoncol.2016.10.005
Liu BL, Robinson M, Han ZQ et al (2003) ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 10:292–303. https://doi.org/10.1038/sj.gt.3301885
Sivendran S, Pan M, Kaufman HL, Saenger Y (2010) Herpes simplex virus oncolytic vaccine therapy in melanoma. Expert Opin Biol Ther 10:1145–1153. https://doi.org/10.1517/14712598.2010.495383
Andtbacka RHI, Collichio F, Harrington KJ, Middleton MR, Downey G, Hrling K, Kaufman HL (2019) Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer 7:145. https://doi.org/10.1186/s40425-019-0623-z
Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S (2010) Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol 17:718–730. https://doi.org/10.1245/s10434-009-0809-6
Audrey-Bayan C, Trager MH, Gartrell-Corrado RD et al (2020) Distinguishing melanophages from tumor in melanoma patients treated with talimogene laherparepvec. Melanoma Res 30:410–415. https://doi.org/10.1097/CMR.0000000000000661
Boni A, Cogdill AP, Dang P et al (2010) Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 70:5213–5219. https://doi.org/10.1158/0008-5472.CAN-10-0118
Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA (2012) Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res : Off J Am Assoc Cancer Res 18:1386–1394. https://doi.org/10.1158/1078-0432.CCR-11-2479
Crespo-Rodriguez E, Bergerhoff K, Bozhanova G et al (2020) Combining BRAF inhibition with oncolytic herpes simplex virus enhances the immune-mediated antitumor therapy of BRAF-mutant thyroid cancer. J Immunother Cancer 8:e000698. https://doi.org/10.1136/jitc-2020-000698
Bommareddy PK, Aspromonte S, Zloza A, Rabkin SD, Kaufman HL (2018) MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Sci Transl Med. 10:417. https://doi.org/10.1126/scitranslmed.aau0417
Roulstone V, Pedersen M, Kyula J et al (2015) BRAF- and MEK-targeted small molecule inhibitors exert enhanced antimelanoma effects in combination with oncolytic reovirus through ER stress. Mol Ther 23:931–942. https://doi.org/10.1038/mt.2015.15
Silva JM, Bulman C, McMahon M (2014) BRAFV600E cooperates with PI3K signaling, independent of AKT, to regulate melanoma cell proliferation. Mol Cancer Res 12:447–463. https://doi.org/10.1158/1541-7786.MCR-13-0224-T
Dankort D, Curley DP, Cartlidge RA et al (2009) Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 41:544–552. https://doi.org/10.1038/ng.356
Meeth K, Wang JX, Micevic G, Damsky W, Bosenberg MW (2016) The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res 29:590–597. https://doi.org/10.1111/pcmr.12498
Dobrovolny PL, Bess D (2011) Optimized PCR-based detection of mycoplasma. J Vis Exp. https://doi.org/10.3791/3057
Miller CG, Krummenacher C, Eisenberg RJ et al (2001) Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol Ther 3:160–168. https://doi.org/10.1006/mthe.2000.0240
Abdoli S, Roohvand F, Teimoori-Toolabi L et al (2019) Cytotoxic effect of dual fluorescent-labeled oncolytic herpes simplex virus type 1 on mouse tumorigenic cell lines. Res Pharm Sci 14:27–35. https://doi.org/10.4103/1735-5362.251850
Ribas A, Dummer R, Puzanov I et al (2017) Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 170(1109–19):e10. https://doi.org/10.1016/j.cell.2017.08.027
Ackerman A, Klein O, McDermott DF et al (2014) Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer 120:1695–1701. https://doi.org/10.1002/cncr.28620
Ribas A, Hodi FS, Lawrence DP et al (2016) Pembrolizumab (pembro) in combination with dabrafenib (D) and trametinib (T) for BRAF-mutant advanced melanoma: phase 1 KEYNOTE-022 study. J Clin Oncol. 34:3014. https://doi.org/10.1200/JCO.2016.34.15_suppl.3014
Pelster MS, Amaria RN (2019) Combined targeted therapy and immunotherapy in melanoma: a review of the impact on the tumor microenvironment and outcomes of early clinical trials. Ther Adv Med Oncol 11:1758835919830826. https://doi.org/10.1177/1758835919830826
Acknowledgments
We would like to thank the Human Immune Monitoring Core (HIMC) and Molecular Pathology Core at Columbia University Irving Medical Center for their help in processing the samples. We would also like to thank the Institute of Comparative Medicine for their help in caring for the animals.
Funding
The authors of this publication were supported by the National Institutes of Health through Grant Numbers R01FD006108, Project 1R01CA260375-01 (Y.M. Saenger), and KL2TR001874 (R.D. Gartrell). F. Lozano is supported by Spanish Ministerio de Economía y Competitividad (MINECO) through grant SAF-2016–80535-R co-financed by European Development Regional Fund. I. Simoes was supported by Fundação para a Ciência e a Tecnologia through a fellowship (SFRH/ BD/75738/2011). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Yvonne Saenger is also supported by an Irving Assistant Professorship at Columbia University’s NIH/NCATS CTSA Program hub: UL1TR001873 and the Amgen-CUMC-MRA (Melanoma Research Alliance) Established Investigator Academic-Industry Partnership Award. Robyn Gartrell is also supported by Swim Across America. The funding sources had no role preparation of the manuscript or the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
RG, ZB, and YS contributed to study design and conception and developed the first draft of the manuscript. ER, RPL, SW, MM, HM, SW, and BF contributed to material preparation and creation of figures and tables. Data collection and analysis were performed by RG, ZB, FL, CC, and YS. RG, ZB, IS, CE, YF, DD, LB, GF, HM, ER, SW, BF, BH, and YS discussed the manuscript sections and contributed with updated references. All authors commented on previous versions of the manuscript and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest:
Authors have no disclosures.
Ethical Approval
All animal experiments were performed in accordance with institutional and national guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at Columbia University Irving Medical Center (CUIMC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gartrell, R.D., Blake, Z., Rizk, E.M. et al. Combination immunotherapy including OncoVEXmGMCSF creates a favorable tumor immune micro-environment in transgenic BRAF murine melanoma. Cancer Immunol Immunother 71, 1837–1849 (2022). https://doi.org/10.1007/s00262-021-03088-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03088-y