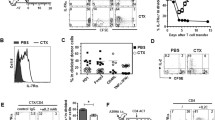
Abstract
Lymphodepleting cytotoxic regimens enhance the antitumor effects of adoptively transferred effector and naïve T cells. Although the mechanisms of antitumor immunity augmentation by lymphodepletion have been intensively investigated, the effects of lymphodepletion followed by T cell transfer on immune checkpoints in the tumor microenvironment remain unclear. The current study demonstrated that the expression of immune checkpoint molecules on transferred donor CD4+ and CD8+ T cells was significantly decreased in lymphodepleted tumor-bearing mice. In contrast, lymphodepletion did not reduce immune checkpoint molecule levels on recipient CD4+ and CD8+ T cells. Administration of anti-PD-1 antibodies after lymphodepletion and adoptive transfer of T cells significantly inhibited tumor progression. Further analysis revealed that transfer of both donor CD4+ and CD8+ T cells was responsible for the antitumor effects of a combination therapy consisting of lymphodepletion, T cell transfer and anti-PD-1 treatment. Our findings indicate that a possible mechanism underlying the antitumor effects of lymphodepletion followed by T cell transfer is the prevention of donor T cell exhaustion and dysfunction. PD-1 blockade may reinvigorate exhausted recipient T cells and augment the antitumor effects of lymphodepletion and adoptive T cell transfer.






Similar content being viewed by others
Availability of data and material
All the data are available under reasonable request. Material requests should be addressed to satoshi7@med.niigata-u.ac.jp.
Abbreviations
- CAR-T:
-
Chimeric antigen receptor T
- DCs:
-
Dendritic cells
- Foxp3:
-
Forkhead box P3
- i.v:
-
Intravenous
- i.p:
-
Intraperitoneally
- ICIs:
-
Immune checkpoint inhibitors
- mAbs:
-
Monoclonal antibodies
- MDSCs:
-
Myeloid-derived suppressor cells
- PD-1:
-
Programmed-cell death-1
- PD-L1:
-
PD-ligand 1
- s.c:
-
Subcutaneous
- TDLNs:
-
Tumor-draining lymph nodes
- TILs:
-
Tumor-infiltrating lymphocytes
References
Wang LX, Shu S, Plautz GE (2005) Host lymphodepletion augments T cell adoptive immunotherapy through enhanced intratumoral proliferation of effector cells. Cancer Res 65:9547–9554. https://doi.org/10.1158/0008-5472.Can-05-1175
North RJ (1982) Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med 155:1063–1074
North RJ (1984) Gamma-irradiation facilitates the expression of adoptive immunity against established tumors by eliminating suppressor T cells. Cancer Immunol Immunother: CII 16:175–181
Restifo NP, Dudley ME, Rosenberg SA (2012) Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12:269–281. https://doi.org/10.1038/nri3191
Rosenberg SA, Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science (New York, N.Y.) 348:62–8. https://doi.org/10.1126/science.aaa4967
Gauthier J, Bezerra ED, Hirayama AV et al (2020) Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B cell malignancies. Blood. https://doi.org/10.1182/blood.2020006770
Baba J, Watanabe S, Saida Y et al (2012) Depletion of radio-resistant regulatory T cells enhances antitumor immunity during recovery from lymphopenia. Blood 120:2417–2427. https://doi.org/10.1182/blood-2012-02-411124
Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN (2002) T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Investig 110:185–192. https://doi.org/10.1172/jci15175
Watanabe S, Arita M, Takahashi M, Saida Y, Koya T, Kikuchi T (2017) Effect of lymphodepletion on donor T Cells and the role of recipient cells persisting after cytotoxic treatments in cancer immunotherapies. Crit Rev Immunol 37:59–73. https://doi.org/10.1615/CritRevImmunol.2018019497
Gattinoni L, Finkelstein SE, Klebanoff CA et al (2005) Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+T cells. J Exp Med 202:907–912. https://doi.org/10.1084/jem.20050732
Klebanoff C, Khong H, Antony P, Palmer D, Restifo N (2005) Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol 26:111–117. https://doi.org/10.1016/j.it.2004.12.003
Kato K, Cho BC, Takahashi M et al (2019) Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:1506–1517. https://doi.org/10.1016/s1470-2045(19)30626-6
Kang YK, Boku N, Satoh T et al (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) 390:2461–2471. https://doi.org/10.1016/s0140-6736(17)31827-5
Ferris RL, Blumenschein G Jr, Fayette J et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856–1867. https://doi.org/10.1056/NEJMoa1602252
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. https://doi.org/10.1056/NEJMoa1504627
Robert C, Long GV, Brady B et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330. https://doi.org/10.1056/NEJMoa1412082
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813. https://doi.org/10.1056/NEJMoa1510665
Gandhi L, Rodriguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/NEJMoa1801005
Sato H, Okonogi N, Nakano T (2020) Rationale of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy for cancer treatment. Int J Clin Oncol 25:801–809. https://doi.org/10.1007/s10147-020-01666-1
Kawazoe A, Fukuoka S, Nakamura Y et al (2020) Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol 21:1057–1065. https://doi.org/10.1016/s1470-2045(20)30271-0
McLane LM, Abdel-Hakeem MS, Wherry EJ (2019) CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 37:457–495. https://doi.org/10.1146/annurev-immunol-041015-055318
Shu SY, Rosenberg SA (1985) Adoptive immunotherapy of newly induced murine sarcomas. Cancer Res 45:1657–1662
Gong J, Chehrazi-Raffle A, Reddi S, Salgia R (2018) Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer 6:8. https://doi.org/10.1186/s40425-018-0316-z
Jiang Y, Li Y, Zhu B (2015) T-cell exhaustion in the tumor microenvironment. Cell Death Dis 6:e1792. https://doi.org/10.1038/cddis.2015.162
Crespo J, Sun H, Welling TH, Tian Z, Zou W (2013) T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol 25:214–221. https://doi.org/10.1016/j.coi.2012.12.003
Zarour HM (2016) Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res : An Off J Am Assoc Cancer Res 22:1856–1864. https://doi.org/10.1158/1078-0432.Ccr-15-1849
Saida Y, Watanabe S, Tanaka T et al (2015) Critical roles of chemoresistant effector and regulatory T cells in antitumor immunity after lymphodepleting chemotherapy. J Immunol 195:726–735. https://doi.org/10.4049/jimmunol.1401468
Kansy BA, Concha-Benavente F, Srivastava RM et al (2017) PD-1 status in CD8(+) T cells associates with survival and Anti-PD-1 therapeutic outcomes in head and neck cancer. Cancer Res 77:6353–6364. https://doi.org/10.1158/0008-5472.Can-16-3167
Kurtulus S, Madi A, Escobar G et al (2019) Checkpoint blockade immunotherapy induces dynamic changes in PD-1(-)CD8(+) tumor-infiltrating T cells. Immunity 50:181–94.e6. https://doi.org/10.1016/j.immuni.2018.11.014
Ueha S, Yokochi S, Ishiwata Y et al (2015) Robust antitumor effects of combined anti-CD4-depleting antibody and anti-PD-1/PD-L1 immune checkpoint antibody treatment in mice. Cancer Immunol Res 3:631–640. https://doi.org/10.1158/2326-6066.Cir-14-0190
Hsu J, Hodgins JJ, Marathe M et al (2018) Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Investig 128:4654–4668. https://doi.org/10.1172/jci99317
Gabrilovich DI (2017) Myeloid-derived suppressor cells. Cancer Immunol Res 5:3–8. https://doi.org/10.1158/2326-6066.Cir-16-0297
Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S (2008) Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol 181:3291–3300. https://doi.org/10.4049/jimmunol.181.5.3291
Tada K, Kitano S, Shoji H et al (2016) Pretreatment immune status correlates with progression-free survival in chemotherapy-treated metastatic colorectal cancer patients. Cancer Immunol Res 4:592–599. https://doi.org/10.1158/2326-6066.CIR-15-0298
Wang LX (2005) Host lymphodepletion augments T cell adoptive immunotherapy through enhanced intratumoral proliferation of effector cells. Cancer Res 65:9547–9554. https://doi.org/10.1158/0008-5472.can-05-1175
Galluzzi L, Senovilla L, Zitvogel L, Kroemer G (2012) The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 11:215–233. https://doi.org/10.1038/nrd3626
Chen G, Emens LA (2013) Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother: CII 62:203–216. https://doi.org/10.1007/s00262-012-1388-0
Thommen DS, Schumacher TN (2018) T Cell Dysfunction in cancer. Cancer Cell 33:547–562. https://doi.org/10.1016/j.ccell.2018.03.012
Kamada T, Togashi Y, Tay C et al (2019) PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A 116:9999–10008. https://doi.org/10.1073/pnas.1822001116
Serpico AF, Visconti R, Grieco D (2020) Exploiting immune-dependent effects of microtubule-targeting agents to improve efficacy and tolerability of cancer treatment. Cell Death Dis 11:361. https://doi.org/10.1038/s41419-020-2567-0
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G (2013) Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39:74–88. https://doi.org/10.1016/j.immuni.2013.06.014
Jure-Kunkel M, Masters G, Girit E, Dito G, Lee F, Hunt JT, Humphrey R (2013) Synergy between chemotherapeutic agents and CTLA-4 blockade in preclinical tumor models. Cancer Immunol Immunother : CII 62:1533–1545. https://doi.org/10.1007/s00262-013-1451-5
Zhang R, Lyu C, Lu W, Pu Y, Jiang Y, Deng Q (2020) Synergistic effect of programmed death-1 inhibitor and programmed death-1 ligand-1 inhibitor combined with chemotherapeutic drugs on DLBCL cell lines in vitro and in vivo. Am J Cancer Res 10:2800–2812
Tanaka H, Matsushima H, Nishibu A, Clausen BE, Takashima A (2009) Dual therapeutic efficacy of vinblastine as a unique chemotherapeutic agent capable of inducing dendritic cell maturation. Cancer Res 69:6987–6994. https://doi.org/10.1158/0008-5472.Can-09-1106
Funding
This work was supported by the Japan Society for Promotion of Science KAKENHI Grant Number 24591157 (to S. Watanabe).
Author information
Authors and Affiliations
Contributions
SW, QZ, BH and LD designed experiments. MT, RS, MA, MS, YS, YA, FS, and SH performed experiments. MT, SW, and KS acquired data. SW and YS analyzed data and generated figures. MT and SW wrote the manuscript. AO, SS, and KN supervised the analysis. KI, RK, NA, YO, MH, TK, and TK discussed the results and conclusions and commented on the manuscript at all stages. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical approval
All animal studies were approved by the Niigata University Institutional Animal Care and Use Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
262_2021_3078_MOESM1_ESM.pdf
Expression of checkpoint receptors after lymphodepletion and T cell transfer.B6 mice were inoculated s.c. with B16F10 tumor cells. These mice were irradiated and reconstituted with spleen cells from Ly5.1 mice 7 days after tumor inoculation or injected i.p. with anti-PD-1 mAb (250 μg per mouse) on days 7 and 14. Tumor tissues were harvested and prepared for FACS analysis on day 21. A-L, The percentage of PD-L1+, PD-1+, TIGIT+, ICOS+, TIM-3+, LAG-3+cells among CD4+T cells are shown. B-L, The percentage of PD-L1+, PD-1+, TIGIT+, ICOS+, TIM-3+, LAG-3+cells among CD8+T cells are presented. Data are shown as mean ± SE (n = 3/group). P values were estimated with the Student’s t test and are shown as *p < 0.05, **p < 0.01 or ***p < 0.001 (PDF 264 KB)
262_2021_3078_MOESM2_ESM.pdf
Expression of checkpoint receptors after lymphodepletion and the transfer of T cells from irradiated hosts.B6 mice were inoculated s.c. with MCA205 tumor cells. These mice were irradiated and reconstituted with spleen cells from Ly5.1 mice 7 days after tumor inoculation or injected i.p. with anti-PD-1 mAb (250 μg per mouse) on days 7 and 14. Donor Ly5.1 mice were irradiated 14 days before the harvest of spleens. Tumor tissues were harvested and prepared for FACS analysis on day 21. A-L, The percentage of PD-L1+, PD-1+, TIGIT+, ICOS+, TIM-3+, LAG-3+cells among CD4+T cells are shown. B-L, The percentage of PD-L1+, PD-1+, TIGIT+, ICOS+, TIM-3+, LAG-3+cells among CD8+T cells are presented. Data are shown as mean ± SE (n = 3/group). P values were estimated with the Student’s t test and are shown as *p < 0.05, **p < 0.01 or ***p < 0.001 (PDF 324 KB)
262_2021_3078_MOESM3_ESM.pdf
Expression of checkpoint receptors in tumor-draining lymph node cells after lymphodepletion and the transfer of T cells.B6 mice were inoculated s.c. with MCA205 tumor cells. These mice were irradiated and reconstituted with spleen cells from Ly5.1 mice 7 days after tumor inoculation or injected i.p. with anti-PD-1 mAb (250 μg per mouse) on days 7 and 14. Tumor-draining lymph nodes were harvested and prepared for FACS analysis on day 21. A-L,The percentage of PD-L1+, PD-1+, TIGIT+, ICOS+, TIM-3+, LAG-3+cells among CD4+T cells are shown. B-L, The percentage of PD-L1+, PD-1+, TIGIT+, ICOS+, TIM-3+, LAG-3+cells among CD8+T cells are presented. Data are shown as mean ± SE (n = 3/group). P values were estimated with the Student’s t test and are shown as *p < 0.05 or **p < 0.01 (PDF 302 KB)
262_2021_3078_MOESM4_ESM.pdf
The combination of lymphodepletion, T cell transfer, anti-PD-1 Abs, and anti-CD25 Abs for advanced skin tumor model. MCA205 tumor-bearing mice were irradiated and adoptively transferred naïve T cells 20 days after tumor inoculation. The mice were treated with anti-CD25 mAbs to deplete Tregs on day 20 and received anti-PD-1 treatment on days 20, 26, and 32. P value was estimated with the Student’s t test (PDF 113 KB)
Rights and permissions
About this article
Cite this article
Takahashi, M., Watanabe, S., Suzuki, R. et al. PD-1 blockade therapy augments the antitumor effects of lymphodepletion and adoptive T cell transfer. Cancer Immunol Immunother 71, 1357–1369 (2022). https://doi.org/10.1007/s00262-021-03078-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03078-0