Abstract
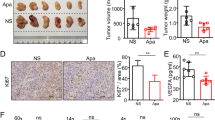
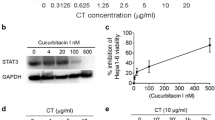
Activation of diacylglycerol kinase alpha (DGKα) augments proliferation and suppresses apoptosis of cancer cells and induces T lymphocyte anergy. We investigated the dual effects of DGKα inhibition on tumorigenesis and anti-tumor immunity with the aim of establishing a novel therapeutic strategy for cancer. We examined the effects of a DGKα inhibitor (DGKAI) on liver cancer cell proliferation and cytokine production by immune cells in vitro and on tumorigenesis and host immunity in a hepatocellular carcinoma (HCC) mouse model. Oral DGKAI significantly suppressed tumor growth and prolonged survival in model mice. Tumor infiltration of T cells and dendritic cells was also enhanced in mice treated with DGKAI, and the production of cytokines and cytotoxic molecules by CD4+ and CD8+ T cells was increased. Depletion of CD8+ T cells reduced the effect of DGKAI. Furthermore, interferon-γ stimulation augmented the expression of programmed cell death-1 ligand (PD-L1) on cancer cells, and DGKAI plus an anti-PD-L1 antibody strongly suppressed the tumor growth. These results suggest that DGKα inhibition may be a promising new treatment strategy for HCC.





Similar content being viewed by others
Availability of data and material
All data used in this study are included in this article and its supplementary files.
Abbreviations
- BMDC:
-
Bone marrow-derived dendritic cell
- DGK:
-
Diacylglycerol kinase
- DGKAI:
-
Diacylglycerol kinase alpha inhibitor
- ERK:
-
Extracellular signal-regulated kinase
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- HCC:
-
Hepatocellular carcinoma
- JNK:
-
C-Jun N-terminal kinase
- OVA:
-
Ovalbumin
- PBMC:
-
Peripheral blood mononuclear cell
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death-1 ligand
- TCR:
-
T cell receptor
- TIL:
-
Tumor-infiltrating lymphocyte
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359-386. https://doi.org/10.1002/ijc.29210
Kudo M, Izumi N, Kubo S, Kokudo N, Sakamoto M, Shiina S, Tateishi R, Nakashima O, Murakami T, Matsuyama Y, Takahashi A, Miyata H, Takayama T (2020) Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res 50(1):15–46. https://doi.org/10.1111/hepr.13438
Kato A, Miyazaki M, Ambiru S, Yoshitomi H, Ito H, Nakagawa K, Shimizu H, Yokosuka O, Nakajima N (2001) Multidrug resistance gene (MDR-1) expression as a useful prognostic factor in patients with human hepatocellular carcinoma after surgical resection. J Surg Oncol 78(2):110–115. https://doi.org/10.1002/jso.1129
Jiang W, Lu Z, He Y, Diasio RB (1997) Dihydropyrimidine dehydrogenase activity in hepatocellular carcinoma: implication in 5-fluorouracil-based chemotherapy. Clin Cancer Res 3(3):395–399
Sakane F, Mizuno S, Komenoi S (2016) Diacylglycerol kinases as emerging potential drug targets for a variety of diseases: an update. Front Cell Dev Biol 4:82. https://doi.org/10.3389/fcell.2016.00082
Yanagisawa K, Yasuda S, Kai M, Imai S, Yamada K, Yamashita T, Jimbow K, Kanoh H, Sakane F (2007) Diacylglycerol kinase alpha suppresses tumor necrosis factor-alpha-induced apoptosis of human melanoma cells through NF-kappaB activation. Biochim Biophys Acta 1771(4):462–474. https://doi.org/10.1016/j.bbalip.2006.12.008
Takeishi K, Taketomi A, Shirabe K, Toshima T, Motomura T, Ikegami T, Yoshizumi T, Sakane F, Maehara Y (2012) Diacylglycerol kinase alpha enhances hepatocellular carcinoma progression by activation of Ras-Raf-MEK-ERK pathway. J Hepatol 57(1):77–83. https://doi.org/10.1016/j.jhep.2012.02.026
Yamaki A, Akiyama R, Murakami C, Takao S, Murakami Y, Mizuno S, Takahashi D, Kado S, Taketomi A, Shirai Y, Goto K, Sakane F (2019) Diacylglycerol kinase α-selective inhibitors induce apoptosis and reduce viability of melanoma and several other cancer cell lines. J Cell Biochem 120(6):10043–10056. https://doi.org/10.1002/jcb.28288
Mérida I, Torres-Ayuso P, Ávila-Flores A, Arranz-Nicolás J, Andrada E, Tello-Lafoz M, Liébana R, Arcos R (2017) Diacylglycerol kinases in cancer. Adv Biol Regul 63:22–31. https://doi.org/10.1016/j.jbior.2016.09.005
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1):23–34. https://doi.org/10.1056/NEJMoa1504030
Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27(4):450–461. https://doi.org/10.1016/j.ccell.2015.03.001
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19(11):1423–1437. https://doi.org/10.1038/nm.3394
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24(5):541–550. https://doi.org/10.1038/s41591-018-0014-x
Jung IY, Kim YY, Yu HS, Lee M, Kim S, Lee J (2018) CRISPR/Cas9-mediated knockout of DGK improves antitumor activities of human T cells. Cancer Res 78(16):4692–4703. https://doi.org/10.1158/0008-5472.Can-18-0030
Arranz-Nicolás J, Ogando J, Soutar D, Arcos-Pérez R, Meraviglia-Crivelli D, Mañes S, Mérida I, Ávila-Flores A (2018) Diacylglycerol kinase α inactivation is an integral component of the costimulatory pathway that amplifies TCR signals. Cancer Immunol Immunother 67(6):965–980. https://doi.org/10.1007/s00262-018-2154-8
Riese MJ, Wang LC, Moon EK, Joshi RP, Ranganathan A, June CH, Koretzky GA, Albelda SM (2013) Enhanced effector responses in activated CD8+ T cells deficient in diacylglycerol kinases. Cancer Res 73(12):3566–3577. https://doi.org/10.1158/0008-5472.Can-12-3874
Sakane F, Yamada K, Kanoh H, Yokoyama C, Tanabe T (1990) Porcine diacylglycerol kinase sequence has zinc finger and E-F hand motifs. Nature 344(6264):345–348. https://doi.org/10.1038/344345a0
Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP (2006) Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol 7(11):1174–1181. https://doi.org/10.1038/ni1400
Riese MJ, Moon EK, Johnson BD, Albelda SM (2016) Diacylglycerol kinases (DGKs): novel targets for improving T cell activity in cancer. Front Cell Dev Biol 4:108. https://doi.org/10.3389/fcell.2016.00108
Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, Burks AW, Zhong XP (2008) Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc Natl Acad Sci USA 105(33):11909–11914. https://doi.org/10.1073/pnas.0711856105
Shulga YV, Topham MK, Epand RM (2011) Regulation and functions of diacylglycerol kinases. Chem Rev 111(10):6186–6208. https://doi.org/10.1021/cr1004106
Toyoshima Y, Kitamura H, Xiang H, Ohno Y, Homma S, Kawamura H, Takahashi N, Kamiyama T, Tanino M, Taketomi A (2019) IL6 modulates the immune status of the tumor microenvironment to facilitate metastatic colonization of colorectal cancer cells. Cancer Immunol Res 7(12):1944–1957. https://doi.org/10.1158/2326-6066.cir-18-0766
Ohno Y, Kitamura H, Takahashi N, Ohtake J, Kaneumi S, Sumida K, Homma S, Kawamura H, Minagawa N, Shibasaki S, Taketomi A (2016) IL-6 down-regulates HLA class II expression and IL-12 production of human dendritic cells to impair activation of antigen-specific CD4(+) T cells. Cancer Immunol Immunother 65(2):193–204. https://doi.org/10.1007/s00262-015-1791-4
Kanada Y (2013) Investigasion of the freely available easy-to-use software ‘EZR’ for medical statictics. Bone Marrow Transplantat 48:452–458. https://doi.org/10.1038/bmt.2021.224
Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M (2020) Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol 72(2):307–319. https://doi.org/10.1016/j.jhep.2019.09.025
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL (2020) Atezolizumab plus Bevacizumab in Unresectable hepatocellular Carcinoma. N Engl J Med 382(20):1894–1905. https://doi.org/10.1056/NEJMoa1915745
Arranz-Nicolás J, Martin-Salgado M, Adán-Barrientos I, Liébana R, Del Carmen M-O, Leitner J, Steinberger P, Ávila-Flores A, Merida I (2021) Diacylglycerol kinase α inhibition cooperates with PD-1-targeted therapies to restore the T cell activation program. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-021-02924-5
Noessner E (2017) DGK-α: a checkpoint in cancer-mediated immuno-inhibition and target for immunotherapy. Front Cell Dev Biol 5:16. https://doi.org/10.3389/fcell.2017.00016
Sun C, Mezzadra R, Schumacher TN (2018) Regulation and function of the PD-L1 checkpoint. Immunity 48(3):434–452. https://doi.org/10.1016/j.immuni.2018.03.014
Yao W, He JC, Yang Y, Wang JM, Qian YW, Yang T, Ji L (2017) The prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a systematic review and meta-analysis. Sci Rep 7(1):7525. https://doi.org/10.1038/s41598-017-08128-1
Liu Z, Zhao Y, Fang J, Cui R, Xiao Y, Xu Q (2017) SHP2 negatively regulates HLA-ABC and PD-L1 expression via STAT1 phosphorylation in prostate cancer cells. Oncotarget 8(32):53518–53530. https://doi.org/10.18632/oncotarget.18591
Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, Vale RD (2017) T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science (New York, NY) 355(6332):1428–1433. https://doi.org/10.1126/science.aaf1292
Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T (2012) Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 209(6):1201–1217. https://doi.org/10.1084/jem.20112741
Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ (2007) Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 27(4):635–646. https://doi.org/10.1016/j.immuni.2007.08.014
Merida I, Graziani A, Sakane F (2017) Editorial: diacylglycerol kinase signalling. Front Cell Dev Biol 5:84. https://doi.org/10.3389/fcell.2017.00084
Ohno Y, Toyoshima Y, Yurino H, Monma N, Xiang H, Sumida K, Kaneumi S, Terada S, Hashimoto S, Ikeo K, Homma S, Kawamura H, Takahashi N, Taketomi A, Kitamura H (2017) Lack of interleukin-6 in the tumor microenvironment augments type-1 immunity and increases the efficacy of cancer immunotherapy. Cancer Sci 108(10):1959–1966. https://doi.org/10.1111/cas.13330
Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I (2016) Dual faces of IFNγ in cancer progression: a role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin Cancer Res 22(10):2329–2334. https://doi.org/10.1158/1078-0432.ccr-16-0224
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571. https://doi.org/10.1038/nature13954
Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim DW, Algazi A, Johnson DB, Liniker E, Ben K, Munhoz R, Rapisuwon S, Gherardini PF, Chmielowski B, Wang X, Shintaku IP, Wei C, Sosman JA, Joseph RW, Postow MA, Carlino MS, Hwu WJ, Scolyer RA, Messina J, Cochran AJ, Long GV, Ribas A (2018) High response rate to PD-1 blockade in desmoplastic melanomas. Nature 553(7688):347–350. https://doi.org/10.1038/nature25187
Kim JM, Chen DS (2016) Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol 27(8):1492–1504. https://doi.org/10.1093/annonc/mdw217
Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M (2015) IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer 112(9):1501–1509. https://doi.org/10.1038/bjc.2015.101
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science (New York, NY) 348(6230):56–61. https://doi.org/10.1126/science.aaa8172
Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay D, Lafdil F, Pawlotsky JM (2016) Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology (Baltimore, MD) 64(6):2038–2046. https://doi.org/10.1002/hep.28710
Dai X, Xue J, Hu J, Yang SL, Chen GG, Lai PBS, Yu C, Zeng C, Fang X, Pan X, Zhang T (2017) Positive expression of programmed death ligand 1 in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. Transl Oncol 10(4):511–517. https://doi.org/10.1016/j.tranon.2017.03.009
Zou W, Wolchok JD, Chen L (2016) PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 8(328):328rv324. https://doi.org/10.1126/scitranslmed.aad7118
Liao H, Chen W, Dai Y, Richardson JJ, Guo J, Yuan K, Zeng Y, Xie K (2019) Expression of programmed cell death-ligands in hepatocellular carcinoma: correlation with immune microenvironment and survival outcomes. Front Oncol 9:883. https://doi.org/10.3389/fonc.2019.00883
Wu K, Kryczek I, Chen L, Zou W, Welling TH (2009) Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7–H1/programmed death-1 interactions. Cancer Res 69(20):8067–8075. https://doi.org/10.1158/0008-5472.can-09-0901
Fu L, Li S, Xiao W, Yu K, Li S, Yuan S, Shen J, Dong X, Fang Z, Zhang J, Chen S, Li W, You H, Xia X, Kang T, Tan J, Chen G, Yang AK, Gao Y, Zhou P (2021) DGKA mediates resistance to PD-1 blockade. Cancer Immunol Res 9(4):371–385. https://doi.org/10.1158/2326-6066.CIR-20-0216
Olmez I, Love S, Xiao A, Manigat L, Randolph P, McKenna BD, Neal BP, Boroda S, Li M, Brenneman B, Abounader R, Floyd D, Lee J, Nakano I, Godlewski J, Bronisz A, Sulman EP, Mayo M, Gioeli D, Weber M, Harris TE, Purow B (2018) Targeting the mesenchymal subtype in glioblastoma and other cancers via inhibition of diacylglycerol kinase alpha. Neuro Oncol 20(2):192–202. https://doi.org/10.1093/neuonc/nox119
Acknowledgements
We thank Dr. T. Kamiyama and members of the Department of Gastroenterological Surgery I, Hokkaido University, for providing clinical samples, Dr. Y. Hatanaka, Dr. K. Hatanaka, Dr. S. Kii, N. Kobayashi, and H. Xiang for technical assistance and thoughtful advice. The authors thank Ono Pharmaceutical Co., Ltd. for the donation of DGKAI and secretarial assistance. The authors thank Susan Furness, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This work was partially supported by a Grant-in-Aid for Scientific Research B (19H03724 to AT) and for Challenging Research (Exploratory) (18K19571 to AT) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (MEXT), by Ono Pharmaceutical Co. Ltd., by Akiyama Life Science Foundation, and by the Joint Research Program of the Institute for Genetic Medicine, Hokkaido University. All funding sources had no involvement in study design and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
NO, YS, KG, FS, HK, AT designed the study. NO, KS, SS, HK collected the data. NO, KS, SS, HK, AT analyzed the data. All authors interpreted the data and prepared and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Consent to participate
Written informed consent was obtained from all participants.
Consent for publication
All mouse experiments were approved by the Animal Ethics Committee of Hokkaido University and conducted in accordance with the institution’s Guide for the Care and Use of Laboratory Animals. The experiments conformed to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Ethics approval
The human study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Boards of Hokkaido University, Hokkaido University Graduate School of Medicine, and the Institute for Genetic Medicine.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Okada, N., Sugiyama, K., Shichi, S. et al. Combination therapy for hepatocellular carcinoma with diacylglycerol kinase alpha inhibition and anti-programmed cell death-1 ligand blockade. Cancer Immunol Immunother 71, 889–903 (2022). https://doi.org/10.1007/s00262-021-03041-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03041-z