Abstract
Background
Cancer cachexia is a multifactorial syndrome characterized by weight loss leading to immune dysfunction that is commonly observed in patients with advanced non-small cell lung cancer (NSCLC). We examined the impact of cachexia on the prognosis of patients with advanced NSCLC receiving pembrolizumab and evaluated whether the pathogenesis of cancer cachexia affects the clinical outcome.
Patients and methods
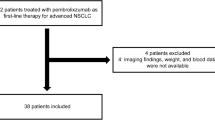
Consecutive patients with advanced NSCLC treated with pembrolizumab were retrospectively enrolled in the study. Serum levels of pro-inflammatory cytokines and appetite-related hormones, which are related to the pathogenesis of cancer cachexia, were analyzed. Cancer cachexia was defined as (1) a body weight loss > 5% over the past 6 months, or (2) a body weight loss > 2% in patients with a body mass index < 20 kg/m2.
Results
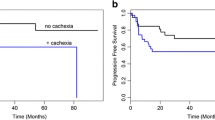
A total of 133 patients were enrolled. Patients with cachexia accounted for 35.3%. No significant difference in the objective response rate was seen between the cachexia and non-cachexia group (29.8% vs. 34.9%, P = 0.550), but the median progression-free survival (PFS) and overall survival (OS) periods were significantly shorter in the cachexia group than in the non-cachexia group (PFS: 4.2 months vs. 7.1 months, P = 0.04, and OS: 10.0 months vs. 26.6 months, P = 0.03). The serum TNF-alpha, IL-1 alpha, IL-8, IL-10, and leptin levels were significantly associated with the presence of cachexia, but not with the PFS or OS.
Conclusion
The presence of cachexia was significantly associated with poor prognosis in advanced NSCLC patients receiving pembrolizumab, not with the response to pembrolizumab.




Similar content being viewed by others
Data availability
Data are available upon reasonable request. De-identified datasets analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALB:
-
Albumin
- ALK:
-
Anaplastic lymphoma kinase
- BMI:
-
Body mass index
- CR:
-
Complete response
- CRP:
-
C-reactive protein
- DOR:
-
Duration of response
- ECOG:
-
Eastern Cooperative Oncology Group
- EGFR:
-
Epidermal growth factor receptor
- GH:
-
Growth hormone
- ICI:
-
Immune checkpoint inhibitor
- IHC:
-
Immunohistochemistry
- IL-1:
-
Interleukin-1
- LDH:
-
Lactate dehydrogenase
- NLR:
-
Neutrophil-to-lymphocyte ratio
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PD-(L)1:
-
Programmed cell death (ligand) 1
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- PS:
-
Performance status
- RECIST:
-
Response evaluation criteria in solid tumors
- TNF-alpha:
-
Tumor necrosis factor-alpha
- TNM:
-
Tumor-node-metastasis
- UICC:
-
Union for International Cancer Control
References
Aoyagi T, Terracina KP, Raza A et al (2015) Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol 7(4):17–29. https://doi.org/10.4251/wjgo.v7.i4.17 [publishedOnlineFirst:2015/04/22]
Argiles JM, Busquets S, Stemmler B et al (2014) Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 14(11):754–762. https://doi.org/10.1038/nrc3829 [publishedOnlineFirst:2014/10/08]
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495. https://doi.org/10.1016/s1470-2045(10)70218-7
Fearon KC, Glass DJ, Guttridge DC (2012) Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 16(2):153–166. https://doi.org/10.1016/j.cmet.2012.06.011 [publishedOnlineFirst:2012/07/17]
Baracos VE, Martin L, Korc M et al (2018) Cancer-associated cachexia. Nat Rev Dis Primers 4:17105. https://doi.org/10.1038/nrdp.2017.105 [publishedOnlineFirst:2018/01/19]
Flint TR, Fearon DT, Janowitz T (2017) Connecting the metabolic and immune responses to cancer. Trends Mol Med 23(5):451–464. https://doi.org/10.1016/j.molmed.2017.03.001 [publishedOnlineFirst:2017/04/12]
Keusch GT, Farthing MJ (1986) Nutrition and infection. Annu Rev Nutr 6:131–154. https://doi.org/10.1146/annurev.nu.06.070186.001023 [publishedOnlineFirst:1986/01/01]
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643 [publishedOnlineFirst:2015/09/29]
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135. https://doi.org/10.1056/NEJMoa1504627 [publishedOnlineFirst:2015/06/02]
Gandhi L, Rodriguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. https://doi.org/10.1056/NEJMoa1801005 [publishedOnlineFirst:2018/04/17]
Reck M, Rodriguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774 [publishedOnlineFirst:2016/10/11]
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet 389(10066):255–265. https://doi.org/10.1016/s0140-6736(16)32517-x
Antonia SJ, Borghaei H, Ramalingam SS et al (2019) Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol 20(10):1395–1408. https://doi.org/10.1016/s1470-2045(19)30407-3
Garon EB, Hellmann MD, Rizvi NA et al (2019) Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 37(28):2518–2527. https://doi.org/10.1200/JCO.19.00934 [publishedOnlineFirst:2019/06/04]
Baracos VE, Reiman T, Mourtzakis M et al (2010) Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr 91(4):1133S-S1137. https://doi.org/10.3945/ajcn.2010.28608C [publishedOnlineFirst:2010/02/19]
Mantovani G, Maccio A, Madeddu C et al (2010) Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 15(2):200–211. https://doi.org/10.1634/theoncologist.2009-0153 [publishedOnlineFirst:2010/02/17]
Suzuki H, Asakawa A, Amitani H et al (2013) Cancer cachexia–pathophysiology and management. J Gastroenterol 48(5):574–594. https://doi.org/10.1007/s00535-013-0787-0 [publishedOnlineFirst:2013/03/21]
Miyawaki T, Naito T, Kodama A et al (2020) Desensitizing effect of cancer cachexia on immune checkpoint inhibitors in patients with advanced NSCLC. JTO Clin Res Rep. https://doi.org/10.1016/j.jtocrr.2020.100020
Roch B, Coffy A, Jean-Baptiste S et al (2020) Cachexia—sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer 143:19–26. https://doi.org/10.1016/j.lungcan.2020.03.003 [publishedOnlineFirst:2020/03/23]
Roach C, Zhang N, Corigliano E et al (2016) Development of a companion diagnostic Pd-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol 24(6):392–397. https://doi.org/10.1097/PAI.0000000000000408 [publishedOnlineFirst:2016/06/23]
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026 [published Online First: 2008/12/23]
Fujitsuka N, Asakawa A, Uezono Y et al (2011) Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry 1:e23. https://doi.org/10.1038/tp.2011.25 [publishedOnlineFirst:2011/01/01]
Gerhardt CC, Romero IA, Cancello R et al (2001) Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol 175(1–2):81–92. https://doi.org/10.1016/s0303-7207(01)00394-x [publishedOnlineFirst:2001/04/28]
Acosta JC, O’Loghlen A, Banito A et al (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133(6):1006–1018. https://doi.org/10.1016/j.cell.2008.03.038
Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME et al (2017) Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev 60:24–31. https://doi.org/10.1016/j.ctrv.2017.08.004
Yuan A, Chen JJ, Yao PL et al (2005) The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci 10:853–865. https://doi.org/10.2741/1579 [publishedOnlineFirst:2004/12/01]
Schalper KA, Carleton M, Zhou M et al (2020) Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med 26(5):688–692. https://doi.org/10.1038/s41591-020-0856-x
Yuen KC, Liu LF, Gupta V et al (2020) High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med 26(5):693–698. https://doi.org/10.1038/s41591-020-0860-1 [publishedOnlineFirst:2020/05/15]
Laino AS, Woods D, Vassallo M et al (2020) Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer. https://doi.org/10.1136/jitc-2020-000842 [published Online First: 2020/06/26]
Johnson G, Salle A, Lorimier G et al (2008) Cancer cachexia: measured and predicted resting energy expenditures for nutritional needs evaluation. Nutrition 24(5):443–450. https://doi.org/10.1016/j.nut.2008.01.013 [publishedOnlineFirst:2008/03/25]
Lundholm K, Gunnebo L, Korner U et al (2010) Effects by daily long term provision of ghrelin to unselected weight-losing cancer patients: a randomized double-blind study. Cancer 116(8):2044–2052. https://doi.org/10.1002/cncr.24917 [publishedOnlineFirst:2010/02/27]
Tschop M, Smiley DL, Heiman ML (2000) Ghrelin induces adiposity in rodents. Nature 407(6806):908–913. https://doi.org/10.1038/35038090 [publishedOnlineFirst:2000/11/01]
Davenport AP, Bonner TI, Foord SM et al (2005) International union of pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev 57(4):541–6. https://doi.org/10.1124/pr.57.4.1 [published Online First: 2005/12/31]
Arora GK, Gupta A, Narayanan S et al (2018) Cachexia-associated adipose loss induced by tumor-secreted leukemia inhibitory factor is counterbalanced by decreased leptin. JCI Insight. https://doi.org/10.1172/jci.insight.121221 [published Online First: 2018/07/27]
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395(6704):763–770. https://doi.org/10.1038/27376 [publishedOnlineFirst:1998/10/31]
Katakami N, Uchino J, Yokoyama T et al (2018) Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 124(3):606–616. https://doi.org/10.1002/cncr.31128 [publishedOnlineFirst:2017/12/06]
Temel JS, Abernethy AP, Currow DC et al (2016) Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 17(4):519–531. https://doi.org/10.1016/s1470-2045(15)00558-6
Proctor MJ, Morrison DS, Talwar D et al (2011) An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer 104(4):726–734. https://doi.org/10.1038/sj.bjc.6606087 [publishedOnlineFirst:2011/01/27]
Pastorino U, Morelli D, Leuzzi G et al (2017) Baseline and postoperative C-reactive protein levels predict mortality in operable lung cancer. Eur J Cancer 79:90–97. https://doi.org/10.1016/j.ejca.2017.03.020 [publishedOnlineFirst:2017/05/05]
Fang S, Wang Y, Sui D et al (2015) C-reactive protein as a marker of melanoma progression. J Clin Oncol 33(12):1389–1396. https://doi.org/10.1200/JCO.2014.58.0209 [publishedOnlineFirst:2015/03/18]
McMillan DC (2008) An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc 67(3):257–262. https://doi.org/10.1017/S0029665108007131 [publishedOnlineFirst:2008/05/03]
Agnoli C, Grioni S, Pala V et al (2017) Biomarkers of inflammation and breast cancer risk: a case-control study nested in the EPIC-Varese cohort. Sci Rep 7(1):12708. https://doi.org/10.1038/s41598-017-12703-x [publishedOnlineFirst:2017/10/07]
Bartlett EK, Flynn JR, Panageas KS et al (2019) High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer 126(1):76–85. https://doi.org/10.1002/cncr.32506
Yoshida T, Ichikawa J, Giuroiu I et al (2020) C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J Immunother Cancer. https://doi.org/10.1136/jitc-2019-000234 [published Online First: 2020/04/19]
Capone M, Giannarelli D, Mallardo D et al (2018) Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J ImmunoTher Cancer. https://doi.org/10.1186/s40425-018-0383-1
Zhang L, Liu SH, Wright TT et al (2015) C-reactive protein directly suppresses Th1 cell differentiation and alleviates experimental autoimmune encephalomyelitis. J Immunol 194(11):5243–5252. https://doi.org/10.4049/jimmunol.1402909 [publishedOnlineFirst:2015/04/29]
Acknowledgements
We thank Shoko Azuhata for her donation to the study and all the patients who contributed to this study. We also thank all the investigators from the oncologic department of the National Cancer Center Hospital, for their participation and contributions.
Funding
Nothing.
Author information
Authors and Affiliations
Contributions
HJ, TY, HH, and SY designed the study, performed data analysis, and wrote the manuscript. HJ, TY, HH, SY, YM, YS, YO, YG, NY, KT, MN, and YO read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
HJ has nothing to disclose. TY reports grants from ONO Pharmaceutical, during the conduct of the study; grants and personal fees from AstraZeneca, grants and personal fees from Bristol Myers Squibb, grants from Takeda, personal fees from Chugai, personal fees from Novartis, grants from MSD, grants from AbbVie, outside the submitted work; HH reports grants and personal fees from BMS, grants and personal fees from MSD, grants and personal fees from Chugai, grants and personal fees from Taiho, grants and personal fees from AstraZeneca, grants from Astellas, grants from Merck Serono, grants from Genomic Health, grants and personal fees from Lilly, grants and personal fees from Ono, outside the submitted work; SY has grants from Nippon Boehringer Ingelheim. YM reports grants from National Cancer Center Research and Development Fund, grants from Grant-in-Aid for Scientific Research on Innovative Areas, grants from Hitachi, Ltd., grants from Hitachi High-Technologies, personal fees from Olympus, personal fees from AstraZeneca, personal fees from Novartis, personal fees from COOK, personal fees from AMCO INC., outside the submitted work; YS has nothing to disclose. YO reports personal fees from AstraZeneca K.K., personal fees from Nippon Boehringer Ingelheim, personal fees from Eli Lilly K.K., personal fees from MSD K.K., personal fees from Chugai Pharma Co., Ltd, personal fees from Ono Pharma Co., Ltd, personal fees from Bristol Myers Squibb, outside the submitted work; YG reports grants and personal fees from Eli Lilly, grants and personal fees from Chugai, grants and personal fees from Taiho Pharmaceutical, personal fees from Boehringer Ingelheim, grants and personal fees from Pfizer, grants and personal fees from Novartis, personal fees from AstraZeneca, grants and personal fees from MSD, grants and personal fees from Guardant Health, grants and personal fees from Ono Pharmaceutical, grants from Kyorin, grants from Daiichi Sankyo, personal fees from Illumina, outside the submitted work; NY reports grants from Chugai, grants from Taiho, grants from Eisai, grants from Lilly, grants from Quintiles, grants from Astellas, grants from BMS, grants from Novartis, grants from Daiichi Sankyo, grants from Pfizer, grants from Boehringer Ingelheim, grants from Kyowa Hakko Kirin, grants from Bayer, grants from ONO PHARMACEUTICAL CO., LTD, grants from Takeda, personal fees from ONO PHARMACEUTICAL CO., LTD, personal fees from Chugai, personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Lilly, personal fees from BMS, personal fees from Eisai, personal fees from Otsuka, personal fees from Takeda, personal fees from Boehringer Ingelheim, personal fees from Cimic, grants from Janssen Pharma, grants from MSD, grants from Merck, personal fees from Sysmex, grants from GSK, grants from Sumitomo Dainippon, outside the submitted work; KT reports grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Nippon Boehringer lngelheim Co., Ltd., grants and personal fees from MSD K.K, grants from Glaxo SmithKline Consumer Healthcare Japan K.K, grants from NIPPON SHINYAKU CO., LTD., grants from TSUMURA & CO., grants and personal fees from Pfizer Inc., personal fees from AstraZeneca K.K, grants and personal fees from TAIHO PHARMACEUTICAL CO., LTD., grants from DAIICHI SANKYO Co., LTD., grants from Astellas Pharma Inc., grants and personal fees from KYORIN Pharmaceutical Co., Ltd., grants from KYOWA Hakko Kirin Co., Ltd., grants from TEIJIN PHARMA LIMITED, grants from Sanofi K.K., grants and personal fees from ONO PHARMACEUTICAL CO., LTD., grants from Shionogi & Co., Ltd., personal fees from Bristol Myers Squibb Company, grants and personal fees from Novartis Pharma K.K, grants and personal fees from Eli Lilly Japan K.K, grants from Actelion Pharmaceuticals Japan Ltd., grants from NIPRO PHARMA CORPORATION, grants from Astellas Pharma lnc., grants from Takeda Pharmaceutical Company Limited., grants from Bayer Yakuhin, Ltd, grants from Torii Pharmaceutical Co., Ltd, personal fees from MSD K.K, personal fees from Meiji Seika Pharma Co, Ltd., outside the submitted work; NM reports grants and personal fees from Ono, personal fees from BMS, personal fees from MSD, personal fees from AstraZeneca, grants and personal fees from Roche Diagnostics, personal fees from Novartis, personal fees from Taiho, personal fees from Chugai, personal fees from Miraca Life Science, personal fees from Beckton Dickinson Japan, personal fees from Covidien Japan Inc, outside the submitted work; YO reports grants and personal fees from AstraZeneca, grants and personal fees from Bristol Myers Squibb, personal fees from Boehringer Ingelheim, grants and personal fees from Chugai, personal fees from Celltrion, personal fees from Amgen, grants and personal fees from Eli Lilly, from Janssen, grants and personal fees from Kyorin, grants and personal fees from Nippon Kayaku, grants and personal fees from Novartis, grants and personal fees from ONO Pharmaceutical, grants and personal fees from Pfizer, grants from Ignyta, grants and personal fees from Taiho, grants and personal fees from Takeda, outside the submitted work.
Consent for publication
Not required.
Ethics approval and Consent to participate
This study was approved by an institutional review board (2015–355).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
262_2021_2997_MOESM1_ESM.pdf
Supplemental Figure 1. Overall survival according to CRP level and NLR (N = 133). Kaplan-Meier curves for overall survival according to (a) CRP level and (b) NLR. CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; NR, not reached. (PDF 102 kb)
Rights and permissions
About this article
Cite this article
Jo, H., Yoshida, T., Horinouchi, H. et al. Prognostic significance of cachexia in advanced non-small cell lung cancer patients treated with pembrolizumab. Cancer Immunol Immunother 71, 387–398 (2022). https://doi.org/10.1007/s00262-021-02997-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02997-2