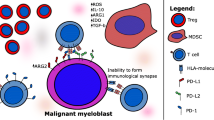
Abstract
Background
Standard care for patients with high-risk myelodysplastic syndrome (MDS) is hypomethylating agents such as azacitidine (AZA), which can induce expression of methylated tumor-associated antigens and therefore potentiate immunotherapeutic targeting.
Method
In this phase 1 trial, we combined AZA with a therapeutic peptide vaccine targeting antigens encoded from NY-ESO-1, MAGE-A3, PRAME, and WT-1, which have previously been demonstrated to be upregulated by AZA treatment.
Result
Five patients who had responded to AZA monotherapy were included in the study and treated with the vaccine. The combination therapy showed only few adverse events during the study period, whereof none classified as serious. However, no specific immune responses could be detected using intracellular cytokine staining or ELISpot assays. Minor changes in the phenotypic composition of immune cells and their expression of stimulatory and inhibitory markers were detected. All patients progressed to AML with a mean time to progression from inclusion (TTP) of 5.2 months (range 2.8 to 7.6). Mean survival was 18.1 months (range 10.9 to 30.6) from MDS diagnosis and 11.3 months (range 4.3 to 22.2) from inclusion. Sequencing of bone marrow showed clonal expansion of malignant cells, as well as appearance of novel mutations.
Conclusion
The patients progressed to AML with an average time of only five months after initiating the combination therapy. This may be unrelated to the experimental treatment, but the trial was terminated early as there was no sign of clinical benefit or immunological response.
Why the manuscript is especially interesting
This study is the first to exploit the potential synergistic effects of combining a multi-peptide cancer vaccine with epigenetic therapy in MDS. Although our results are negative, they emphasize challenges to induce immune reactivity in patients with high-risk MDS.



Similar content being viewed by others
Availability of data and material
Case report forms, trial master files, and experimental data are stored in accordance with EU regulation.
Code availability
R code for experimental statistical analysis is available upon request.
References
Zeidan AM, Shallis RM, Wang R, Davidoff A, Ma X (2019) Epidemiology of myelodysplastic syndromes: why characterizing the beast is a prerequisite to taming it. Blood Rev 34:1–15
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A et al (2009) Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 10:223–232
Bender CM, Pao MM, Jones PA (1998) Inhibition of DNA methylation by 5-Aza-2’-deoxycytidine suppresses the growth of human tumor cell lines. Cancer Res 58:95–101
Ørskov AD, Grønbæk K (2017) DNA methyltransferase Inhibitors in myeloid cancer: clonal eradication or clonal differentiation? Cancer J 23:277–285
Mund C, Hackanson B, Stresemann C, Lübbert M, Lyko F (2005) Characterization of DNA demethylation effects induced by 5-Aza-2′-deoxycytidine in patients with myelodysplastic syndrome. Cancer Res 65:7086–7090
Fonsatti E, Nicolay HJM, Sigalotti L, Calabrò L, Pezzani L, Colizzi F et al (2007) Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2′-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clin Cancer Res 13:3333–3338
Zhou J, Yao Y, Shen Q, Li G, Hu L, Zhang X (2017) Demethylating agent decitabine disrupts tumor-induced immune tolerance by depleting myeloid-derived suppressor cells. J Cancer Res Clin Oncol 143:1371–1380
Chiappinelli KB, Strissel PL, Desrichard A, Chan T, Baylin SB, Correspondence S (2015) Inhibiting DNA methylation causes an interferon response in cancer via dsrna including endogenous retroviruses. Cell 162:974–986
Almstedt M, Blagitko-Dorfs N, Duque-Afonso J, Karbach J, Pfeifer D, Jäger E et al (2010) The DNA demethylating agent 5-aza-2’-deoxycytidine induces expression of NY-ESO-1 and other cancer/testis antigens in myeloid leukemia cells. Leuk Res 34:899–905
Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G et al (2010) Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood 116:1908–1918
Siebenkäs C, Chiappinelli KB, Guzzetta AA, Sharma A, Jeschke J, Vatapalli R et al (2017) Inhibiting DNA methylation activates cancer testis antigens and expression of the antigen processing and presentation machinery in colon and ovarian cancer cells. PLoS ONE 12:e0179501
Srivastava P, Paluch BE, Matsuzaki J, James SR, Collamat-Lai G, Blagitko-Dorfs N et al (2016) Induction of cancer testis antigen expression in circulating acute myeloid leukemia blasts following hypomethylating agent monotherapy. Oncotarget 7:12840–12856
Gang AO, Frøsig TM, Brimnes MK, Lyngaa R, Treppendahl MB, Grønbæk K et al (2014) 5-Azacytidine treatment sensitizes tumor cells to T-cell mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies. Blood Cancer J 4:e197
Saleh MH, Wang L, Goldberg MS (2016) Improving cancer immunotherapy with DNA methyltransferase inhibitors. Cancer Immunol Immunother 65:787–796
Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K et al (2007) Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood 109:1103–1112
Mischo A, Kubuschok B, Ertan K, Preuss K-D, Romeike B, Regitz E et al (2006) Prospective study on the expression of cancer testis genes and antibody responses in 100 consecutive patients with primary breast cancer. Int J Cancer 118:696–703
Goodyear O, Piper K, Khan N, Starczynski J, Mahendra P, Pratt G et al (2005) CD8+ T cells specific for cancer germline gene antigens are found in many patients with multiple myeloma, and their frequency correlates with disease burden. Blood 106:4217–4224
Baumgaertner P, Costa Nunes C, Cachot A, Maby-El Hajjami H, Cagnon L, Braun M et al (2016) Vaccination of stage III/IV melanoma patients with long NY-ESO-1 peptide and CpG-B elicits robust CD8 + and CD4 + T-cell responses with multiple specificities including a novel DR7-restricted epitope. Oncoimmunology. 5:e1216290
Connerotte T, Pel AV, Godelaine D, Tartour E, Schuler-Thurner B, Lucas S et al (2008) Functions of anti-MAGE T-cells induced in melanoma patients under different vaccination modalities. Cancer Res 68:3931–3940
Jäger E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D et al (2006) Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci 103:14453–14458
Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC et al (2015) A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother 64:1251–1260
Griffiths EA, Srivastava P, Matsuzaki J, Brumberger Z, Wang ES, Kocent J et al (2017) NY-ESO-1 vaccination in combination with decitabine induces antigen-specific T-lymphocyte responses in patients with myelodysplastic syndrome. Clin Cancer Res 24:1019–1029
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F et al (2012) Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120:2454–2465
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD et al (2006) Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood 108:419–425
Zarour HM, Maillere B, Brusic V, Coval K, Williams E, Pouvelle-Moratille S et al (2002) NY-ESO-1 119–143 Is a promiscuous major histocompatibility complex class II T-helper epitope recognized by Th1- and Th2-type tumor-reactive CD4+ T Cells. Cancer Res 62:213–218
Neek M, Tucker JA, Kim TI, Molino NM, Nelson EL, Wang SW (2018) Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials 156:194–203
Kessler JH, Beekman NJ, Bres-Vloemans SA, Verdijk P, van Veelen PA, Kloosterman-Joosten AM et al (2001) Efficient identification of novel hla-A*0201–presented cytotoxic t lymphocyte epitopes in the widely expressed tumor antigen prame by proteasome-mediated digestion analysis. J Exp Med 193:73–88
Quintarelli C, Dotti G, Hasan ST, Angelis BD, Hoyos V, Errichiello S et al (2011) High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood 117:3353–3362
Zhang H-G, Chen H-S, Peng J-R, Shang X-Y, Zhang J, Xing Q et al (2007) Specific CD8+ T cell responses to HLA-A2 restricted MAGE-A3 p271–279 peptide in hepatocellular carcinoma patients without vaccination. Cancer Immunol Immunother 56:1945–1954
Liu H, Zha Y, Choudhury N, Malnassy G, Fulton N, Green M et al (2018) WT1 peptide vaccine in montanide in contrast to poly ICLC, is able to induce WT1-specific immune response with TCR clonal enrichment in myeloid leukemia. Exp Hematol Oncol 7:1
Doubrovina E, Carpenter T, Pankov D, Selvakumar A, Hasan A, O’Reilly RJ (2012) Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1+ leukemias. Blood 120:1633–1646
Ueda Y, Ogura M, Miyakoshi S, Suzuki T, Heike Y, Tagashira S et al (2017) Phase 1/2 study of the WT1 peptide cancer vaccine WT4869 in patients with myelodysplastic syndrome. Cancer Sci 108:2445–2453
Oka Y, Tsuboi A, Nakata J, Nishida S, Hosen N, Kumanogoh A et al (2017) Wilms’ tumor gene 1 (WT1) peptide vaccine therapy for hematological malignancies: from CTL epitope identification to recent progress in clinical studies including a cure-oriented strategy. Oncol Res Treat 40:682–690
Uttenthal B, Martinez-Davila I, Ivey A, Craddock C, Chen F, Virchis A et al (2014) Wilms’ tumour 1 (WT1) peptide vaccination in patients with acute myeloid leukaemia induces short-lived WT1-specific immune responses. Br J Haematol 164:366–375
Brayer J, Lancet JE, Powers J, List A, Balducci L, Komrokji R et al (2015) WT1 vaccination in AML and MDS: a pilot trial with synthetic analog peptides. Am J Hematol 90:602–607
Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al (2019) Welcome to the Tidyverse. J Open Source Softw 4:1686
Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R et al (2019) Varsome: the human genomic variant search engine. Bioinformatics 35:1978–1980
Oka S, Ono K, Nohgawa M (2020) The acquisition of trisomy 8 associated with behçet’s-like disease in myelodysplastic syndrome. Leuk Res Rep 13:100196
Fox CB, Haensler J (2013) An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev Vaccines 12:747–758
Aucouturier J, Ascarateil S, Dupuis L (2006) The use of oil adjuvants in therapeutic vaccines. Vaccine 24:S44–S45
van de Loosdrecht AA, van Wetering S, Santegoets SJAM, Singh SK, Eeltink CM, den Hartog Y et al (2018) A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunol Immunother 67:1505–1518
Anguille S, Van de Velde AL, Smits EL, Van Tendeloo VF, Juliusson G, Cools N et al (2017) Dendritic cell vaccination as post-remission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 130:1713–1721
López AMH, Chen-Liang TH, Zurdo M, Carrillo-Tornel S, Panadero J, Salido EJ et al (2020) Cancer testis antigens in myelodysplastic syndromes revisited: a targeted RNA-seq approach. OncoImmunology 9:1824642
Leeuwen-Kerkhoff N van, Westers TM, Poddighe PJ, Povoleri GAM, Timms JA, Kordasti S et al (2021) Reduced frequencies and functional impairment of dendritic cell subsets and non-classical monocytes in myelodysplastic syndromes. Haematologica. https://doi.org/10.3324/haematol.2020.268136
Pleyer L, Valent P, Greil R (2016) Mesenchymal stem and progenitor cells in normal and dysplastic hematopoiesis—masters of survival and clonality? Int J Mol Sci 17:1009
Jurtz V, Paul S, Andreatta M, Marcatili P, Peters B, Nielsen M (2017) NetMHCpan-4.0: improved peptide–MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J Immunol 199:3360–3368
Rezvani K, Yong ASM, Mielke S, Jafarpour B, Savani BN, Le RQ et al (2011) Repeated PR1 and WT1 peptide vaccination in montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica 96:432–440
Ørskov AD, Treppendahl MB, Skovbo A, Holm MS, Friis LS, Hokland M et al (2015) Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS / AML patients: a rationale for combined targeting of PD-1 and DNA methylation. Oncotarget 6:9612–9626
Di Stasi A, Jimenez AM, Minagawa K, Al-Obaidi M, Rezvani K (2015) Review of the results of WT1 peptide vaccination strategies for myelodysplastic syndromes and acute myeloid leukemia from nine different studies. Front Immunol 6:36
Kjeldsen JW, Iversen TZ, Engell-Noerregaard L, Mellemgaard A, Andersen MH, Svane IM (2018) Durable clinical responses and long-term follow-up of stage III–IV non-small-cell lung cancer (NSCLC) patients treated with ido peptide vaccine in a phase i study—a brief research report. Front Immunol 9:2145
Funding
Financial support to this study was provided by the Danish Cancer Society, grant no. R72-A4531 and R146-A9531-16-S2; Herlev-Gentofte hospital research grant and from an unrestricted research grant from Celgene inc.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
Celgene provided an unrestricted research grant for this clinical trial. They were allowed to read the final manuscript prior to publication but did not have any influence on the planning, conduction, or publishing of this clinical trial. There were no other conflicts of interest to report.
Ethics approval
The study was conducted in accordance with the Helsinki Declaration and ICH-GCP, and approved by the Danish Medicines Agency and the regional research ethics committee in Denmark. The trial is registered in the EU Clinical Trials Register (2014–002432-14) and clinicaltrials.gov (NCT02750995).
Consent to participate
Written informed consent was obtained from all patients in the clinical trial following oral and written information.
Consent for publication
All patients consented to the publication of anonymized data in scientific journals prior to receiving written and oral information hereof.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sine Reker Hadrup and Daniel El Fassi are shared senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Holmberg-Thydén, S., Dufva, I.H., Gang, A.O. et al. Epigenetic therapy in combination with a multi-epitope cancer vaccine targeting shared tumor antigens for high-risk myelodysplastic syndrome - a phase I clinical trial. Cancer Immunol Immunother 71, 433–444 (2022). https://doi.org/10.1007/s00262-021-02993-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02993-6