Abstract
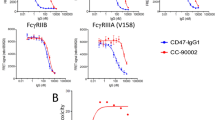
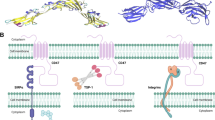
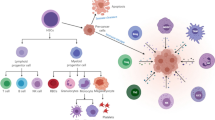
CD47 is a widely expressed cell-surface protein that regulates phagocytosis mediated by cells of the innate immune system, such as macrophages and dendritic cells. CD47 serves as the ligand for a receptor on these innate immune cells, signal regulatory protein (SIRP)-α, which in turn inhibits phagocytosis. Several targeted CD47 therapeutic antibodies have been investigated clinically; however, how to improve its therapeutic efficacy remains unclear. Herein, we developed a CD47 blocking antibody, named IBI188, that could specifically block the CD47-SIRP-α axis, which transduces the "don’t eat me" signal to macrophages. In vitro phagocytosis assays demonstrated the pro-phagocytosis ability of IBI188. Furthermore, several in vivo models were chosen to evaluate the anti-tumor efficacy of IBI188. IBI188 treatment upregulated cell movement- and inflammation-related genes in macrophages. Synergism was observed when combined with an anti-CD20 therapeutic antibody, whose function depends on antibody-dependent cellular cytotoxicity/phagocytosis (ADCC/ADCP). CD47 expression was evaluated following azacytidine (AZA) treatment, a standard-of-care for patients with multiple myeloma; enhanced anti-tumor efficacy was observed in the combination group in AML xenograft models. Notably, IBI188 treatment increased vascular endothelial growth factor-A (VEGF-A) levels in a solid tumor model, and combined treatment with an anti-VEGF-A antibody and IBI188 resulted in an enhanced anti-tumor effect. These data indicate that IBI188 is a therapeutic anti-CD47 antibody with anti-tumor potency, which can be enhanced when used in combination with standard-of-care drugs for cancer treatment.





Similar content being viewed by others
Abbreviations
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- ADCP:
-
Antibody-dependent cellular phagocytosis
- AZA:
-
Azacytidine
- IBI188:
-
A fully human anti-CD47 therapeutic antibody
- VEGF-A:
-
Vascular endothelial growth factor A
References
Vokes EE, Ready N, Felip E et al (2018) Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 29:959–965. https://doi.org/10.1093/annonc/mdy041
El-Khoueiry AB, Sangro B, Yau T et al (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389:2492–2502. https://doi.org/10.1016/S0140-6736(17)31046-2
Jaiswal S, Jamieson CHM, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL (2009) CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138:271–285. https://doi.org/10.1016/j.cell.2009.05.046
Chao MP, Alizadeh AA, Tang C et al (2010) Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142:699–713. https://doi.org/10.1016/j.cell.2010.07.044
Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr, van Rooijen N, Weissman IL (2009) CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138:286–299. https://doi.org/10.1016/j.cell.2009.05.045
Willingham SB, Volkmer J-P, Gentles AJ et al (2012) The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci 109:6662–6667. https://doi.org/10.1073/pnas.1121623109
Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, Park CY, Weissman IL, Majeti R (2011) Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res 71:1374–1384. https://doi.org/10.1158/0008-5472.CAN-10-2238
Li Y, Zhang M, Wang X, Liu W, Wang H, Yang Y-G (2020) Vaccination with CD47 deficient tumor cells elicits an antitumor immune response in mice. Nat Commun. https://doi.org/10.1038/s41467-019-14102-4
Vonderheide RH (2015) CD47 blockade as another immune checkpoint therapy for cancer. Nat Med 21:1122–1123. https://doi.org/10.1038/nm.3965
Ferrara N (2010) Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell 21:687–690. https://doi.org/10.1091/mbc.E09-07-0590
Ferrara N (2010) Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nat Med 16:1107–1111. https://doi.org/10.1038/nm1010-1107
Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9:685–693. https://doi.org/10.1038/nm0603-685
Basu A, Hoerning A, Datta D, Edelbauer M, Stack MP, Calzadilla K, Pal S, Briscoe DM (2010) Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-gamma production. J Immunol 184:545–549. https://doi.org/10.4049/jimmunol.0900397
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2:1096–1103. https://doi.org/10.1038/nm1096-1096
Ziogas AC, Gavalas NG, Tsiatas M et al (2012) VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer 130:857–864. https://doi.org/10.1002/ijc.26094
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. https://doi.org/10.1093/bioinformatics/bts635
Anders S, Pyl PT, Huber W (2015) HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. https://doi.org/10.1093/bioinformatics/btu638
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. https://doi.org/10.1186/s13059-014-0550-8
da Huang W, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. https://doi.org/10.1093/nar/gkn923
da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. https://doi.org/10.1038/nprot.2008.211
Veillette A, Chen J (2018) SIRPα-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol 39:173–184. https://doi.org/10.1016/j.it.2017.12.005
Trepicchio WL, Wang L, Bozza M, Dorner AJ (1997) IL-11 regulates macrophage effector function through the inhibition of nuclear factor-kappaB. J Immunol 159:5661–5670
Shiraki A, Kotooka N, Komoda H, Hirase T, Oyama JI, Node K (2016) Pentraxin-3 regulates the inflammatory activity of macrophages. Biochem Biophys Rep 5:290–295. https://doi.org/10.1016/j.bbrep.2016.01.009
Bai J, Liu Z, Xu Z et al (2015) Epigenetic downregulation of SFRP4 contributes to epidermal hyperplasia in psoriasis. J Immunol 194:4185–4198. https://doi.org/10.4049/jimmunol.1403196
May RC, Machesky LM (2001) Phagocytosis and the actin cytoskeleton. J Cell Sci 114:1061–1077
Sockolosky JT, Dougan M, Ingram JR, Ho CC, Kauke MJ, Almo SC, Ploegh HL, Garcia KC (2016) Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci USA 113:E2646–E2654. https://doi.org/10.1073/pnas.1604268113
Weiskopf K, Ring AM, Ho CCM et al (2013) Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science 341:88–91. https://doi.org/10.1126/science.1238856
Kwong LS, Brown MH, Barclay AN, Hatherley D (2014) Signal-regulatory protein α from the NOD mouse binds human CD47 with an exceptionally high affinity– implications for engraftment of human cells. Immunology 143:61–67. https://doi.org/10.1111/imm.12290
Ho CCM, Guo N, Sockolosky JT, Ring AM, Weiskopf K, Özkan E, Mori Y, Weissman IL, Garcia KC (2015) “Velcro” engineering of high affinity CD47 ectodomain as signal regulatory protein α (SIRPα) antagonists that enhance antibody-dependent cellular phagocytosis. J Biol Chem 290:12650–12663. https://doi.org/10.1074/jbc.M115.648220
Pietsch EC, Dong J, Cardoso R et al (2017) Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J. https://doi.org/10.1038/bcj.2017.7
Feng D, Gip P, McKenna KM et al (2018) Combination treatment with 5F9 and azacitidine enhances phagocytic elimination of acute myeloid leukemia. Blood. https://doi.org/10.1182/blood-2018-99-120170
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Author information
Authors and Affiliations
Contributions
BP, HB and JG provided the yeast platform; ZW performed the affinity measurement experiments; SZ and LW designed and prepared all the needed protein; HN, XG, YG and HJ designed and performed the in vitro assays; LC, MW, YL, JD, PZ, YZ, WW and BC designed and performed all the in vivo mouse experiment; YX and JS performed the bioinformatics analysis, MY, WW and JL designed the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
Bianka Prinz, Hemanta Baruah and James Geoghegan have no potential conflicts of interest to disclose. All other authors are employees of Innovent Biologics (Suzhou).
Ethical approval
All mice were maintained under pathogen-free conditions in the Experimental Animal Center of Innovent Biologics Co., Ltd. (Suzhou, China). All mice-related experiments were approved by the Animal Use and Care Committee of Innovent Biologics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ni, H., Cao, L., Wu, Z. et al. Combined strategies for effective cancer immunotherapy with a novel anti-CD47 monoclonal antibody. Cancer Immunol Immunother 71, 353–363 (2022). https://doi.org/10.1007/s00262-021-02989-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02989-2