Abstract
Introduction
TCR and BCR repertoire diversity plays a critical role in tumor immunity. Thus, analysis of TCR and BCR repertoires might help predict the clinical efficacy of anti-PD-1 treatment.
Methods
Blood samples from 30 patients with non-small cell lung cancer (NSCLC) treated with anti-PD-1 antibody were collected before and six weeks after treatment initiation. The clinical significance of TCR and BCR repertoire diversity in peripheral blood was evaluated in all the enrolled patients (n = 30) or in the subset with (n = 10) or without (n = 20) EGFR/ALK mutation.
Results
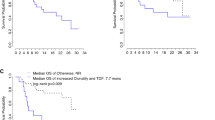
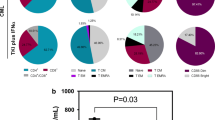
TCR and BCR diversity was significantly correlated at baseline (R = 0.65; P = 1.6 × 10–4) and on treatment (R = 0.72; P = 1.2 × 10–5). Compared to non-responders (SD or PD), responders (PR) showed significantly decreased TCR and BCR diversity after treatment in the EGFR/ALK wild-type subset (P = 0.0014 and P = 0.034, respectively), but not in all the enrolled patients. The post-treatment reduction in TCR and BCR repertoire diversity was also significantly associated with the occurrence of adverse events in the EGFR/ALK wild-type subset (P = 0.022 and P = 0.014, respectively). Patients with more reduced TCR diversity showed better progression-free survival (PFS) in the EGFR/ALK wild-type subset (P = 0.011) but not in the mutant subset.
Conclusions
These findings suggest the clinical significance of changes in peripheral TCR and BCR repertoire diversity after anti-PD-1 treatment in patients with NSCLC without EGFR/ALK mutation. Monitoring of the peripheral TCR and BCR repertoires may serve as a surrogate marker for the early detection of EGFR/ALK wild-type NSCLC patients who would benefit from anti-PD-1 treatment.






Similar content being viewed by others
Code availability
Not applicable.
Abbreviations
- AE:
-
Adverse event
- ALK:
-
Anaplastic lymphoma kinase
- BCR:
-
B cell receptor
- CT:
-
Computed tomography
- ICI:
-
Immune checkpoint inhibitor
- InvSimp index:
-
Inverse Simpson's index
- NSCLC:
-
Non-small cell lung cancer
- PBMCs:
-
Peripheral blood mononuclear cells
- PD:
-
Progressive disease
- PD-1:
-
Programmed cell death protein 1
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- RG:
-
Repertoire Genesis
- SD:
-
Stable disease
- S–W index:
-
Shannon–Weaver index
- TLS:
-
Tertiary lymphoid structures
- TCR:
-
T cell receptor
References
Fitzmaurice C, Abate D, Abbasi N et al (2019) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol 5(12):1749–1768
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454
Brahmer JR, Tykodi SS, Chow LQ et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366(26):2455–2465
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348(6230):56–61
Topalian SL, Taube JM, Anders RA, Pardoll DM (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16(5):275–287
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. New Engl J Med 373(2):123–135
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New Engl J Med 373(17):1627–1639
Herbst RS, Baas P, Kim DW et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Garon EB, Rizvi NA, Hui R et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. New Engl J Med 372(21):2018–2028
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med 375(19):1823–1833
Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL (2015) Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol 42(4):587–600
Han J, Duan J, Bai H et al (2020) TCR repertoire diversity of peripheral PD-1 + CD8 + T cells predicts clinical outcomes after immunotherapy in patients with non-small cell lung cancer. Cancer Immunol Res 8(1):146–154
Zhang J, Ji Z, Caushi JX et al (2020) Compartmental analysis of T-cell clonal dynamics as a function of pathologic response to neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Clin Cancer Res 26(6):1327–1337
Hogan SA, Courtier A, Cheng PF et al (2019) Peripheral blood TCR repertoire profiling may facilitate patient stratification for immunotherapy against melanoma. Cancer Immunol Res 7(1):77–85
Fairfax BP, Taylor CA, Watson RA et al (2020) Peripheral CD8+ T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat Med 26(2):193–199
Valpione S, Galvani E, Tweedy J et al (2020) Immune-awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nat Cancer 1(2):210–221
Snyder A, Nathanson T, Funt SA et al (2017) Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: an exploratory multi-omic analysis. PLoS Med 14(5):e1002309
Kitaura K, Shini T, Matsutani T, Suzuki R (2016) A new high-throughput sequencing method for determining diversity and similarity of T cell receptor (TCR) α and β repertoires and identifying potential new invariant TCR α chains. BMC Immunol 17(1):38
Kitaura K, Yamashita H, Ayabe H, Shini T, Matsutani T, Suzuki R (2017) Different somatic hypermutation levels among antibody subclasses disclosed by a new next-generation sequencing-based antibody repertoire analysis. Front Immunol 8:389
Shannon CE, Weaver W (1949) The mathematical theory of communication. The University of Illinois Press, Champaign
Simpson EH (1949) Measurement of diversity. Nature 163:688
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
R Development Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna. Available at: http://www.R-project.org/.
Gainor JF, Shaw AT, Sequist LV et al (2016) EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 22(18):4585–4593
Calles A, Riess JW, Brahmer JR (2020) Checkpoint blockade in lung cancer with driver mutation: choose the road wisely. Am Soc Clin Oncol Educ Book 40:372–384
Yost KE, Satpathy AT, Wells DK et al (2019) Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med 25(8):1251–1259
Wu TD, Madireddi S, de Almeida PE et al (2020) Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature 579(7798):274–278
Toki MI, Mani N, Smithy JW et al (2018) Immune marker profiling and programmed death ligand 1 expression across NSCLC mutations. J Thorac Oncol 13(12):1884–1896
Simoni Y, Becht E, Fehlings M et al (2018) Bystander CD8 + T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557(7706):575–579
Das R, Bar N, Ferreira M et al (2018) Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 128(2):715–720
Xiao X, Lao XM, Chen MM et al (2016) PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov 6(5):546–559
Cabrita R, Lauss M, Sanna A et al (2020) Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577(7791):561–565
Helmink BA, Reddy SM, Gao J et al (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577(7791):549–555
Petitprez F, de Reyniès A, Keung EZ et al (2020) B cells are associated with survival and immunotherapy response in sarcoma. Nature 577(7791):556–560
Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM (2020) B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol 20(5):294–307
Haratani K, Hayashi H, Chiba Y et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4(3):374–378
Cortellini A, Chiari R, Ricciuti B et al (2019) Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer 20(4):237-247.e231
Weinmann SC, Pisetsky DS (2019) Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford) 58(Suppl 7):59–67
Ramos-Casals M, Brahmer JR, Callahan MK et al (2020) Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 6(1):38
Acknowledgements
We would like to thank Junya Otake (Kanagawa Cancer Center Research Institute) for sample handling and data acquisition.
Funding
This study was supported by AMED under Grant Number JP20ae0101076 (TS, KA, HS, KY) and JSPS KAKENHI Grant Number JP18K19490 (TS).
Author information
Authors and Affiliations
Contributions
KA and TS designed the study. HS, KY, KA and TS obtained financial support for this study. YN, YI and HH contributed to data acquisition. NM, TH and KA collected patient samples and completed the follow-up. YN, TM, YI and KM conducted statistical analyses. YN, TM, YI, NM, HS, KY, KM, KA and TS analyzed and interpreted the data. YN, TM and TS wrote the manuscript, and NM and KA provided critical revisions of the manuscript. All authors approved the final version of the manuscript. YN, TM, YI and NM contributed equally as first authors.
Corresponding author
Ethics declarations
Conflicts of interest
TM is an employee of Repertoire Genesis, Inc. YN has received personal fees from MSD, Ono, Chugai, Eli Lilly, Bristol-Myers Squibb and Nippon Boehringer Ingelheim, and grants from Takeda, Bristol-Myers Squibb and Eli Lilly. HS has received personal fees from Ono, Nippon Boehringer Ingelheim and Novartis, and grants from Chugai, AstraZeneca and MSD. KY has received personal fees from Ono, Chugai and Bristol-Myers Squibb. KA has received grants and personal fees from AstraZeneca, MSD, Bristol Myers Squibb, Ono and Chugai. TS has received grants from BrightPath Biotherapeutics. The other authors have declared that no conflict of interest exists.
Ethics approval and consent to participate
The Institutional Review Board of Kurume University approved the study protocol (Approval number: Kurume University 15210). Written informed consent was received from all participants prior to inclusion in the study.
Consent for publication
Not applicable.
Availability of data and material
The datasets used and analyzed during the current study are available on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakahara, Y., Matsutani, T., Igarashi, Y. et al. Clinical significance of peripheral TCR and BCR repertoire diversity in EGFR/ALK wild-type NSCLC treated with anti-PD-1 antibody. Cancer Immunol Immunother 70, 2881–2892 (2021). https://doi.org/10.1007/s00262-021-02900-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02900-z