Abstract
Introduction
Although recent clinical trials have demonstrated the efficacy of CD19-directed chimeric antigen receptor (CAR) T-cell therapy for refractory or relapsed B acute lymphoblastic leukemia (r/r B-ALL), most trials exclude patients with high-burden CNS leukemia (CNSL) to avoid the risk of severe neurotoxicity. There were only sparse cases describing the effect of CAR T cells on low-burden CNSL, and the safety and effectiveness of CAR T cells in high-burden CNSL remains unknown.
Methods
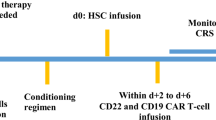
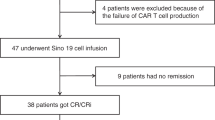
Here, we retrospectively analyzed the results of CD19 CAR T-cell therapy in 12 pediatric patients that had low (Blasts < 20/μL in CSF) or high-burdens (Blasts or intracranial solid mass) of CNS B-ALL, that are enrolled in three clinical trials and one pilot study at Beijing Boren Hospital
Results
Eleven patients (91.7%) achieved complete remission (CR) on day 30, and one patient got CR on day 90 after infusion. Most patient experienced mild cytokine-release syndrome. However, of the five patients who retained > 5/μL blasts in CSF or a solid mass before CAR T-cell expansion, four developed severe (grade 3–4) neurotoxicity featured by persistent cerebral edema and seizure, and they fully recovered after intensive managements. Sustained remission was achieved in 9 of the 12 patients, resulted in a 6-month leukemia-free survival rate of 81.8% (95% CI 59.0–100). Only one patient has CNS relapse again.
Conclusion
Our study demonstrates that CAR T cells are effective in clearing both low- and high-burden CNSL, but a high CNSL burden before CAR T-cell expansion may cause severe neurotoxicity requiring intense intervention.






Similar content being viewed by others
Abbreviations
- Allo-HCT:
-
Allo-hematopoietic stem-cell transplant
- Ara-C:
-
Cytarabine
- CAR:
-
Chimeric antigen receptor
- CNS:
-
Central nervous system
- CNSL:
-
Central nervous system leukemia
- CR:
-
Complete remission
- CRS:
-
Cytokine release syndrome
- CSF:
-
Cerebrospinal fluid
- Dex:
-
Dexamethasone
- EMDs:
-
Extramedullary diseases
- ICANS:
-
Immune effector cell-associated neurotoxicity syndrome
- IDA:
-
Darubicin
- IL-6:
-
Interleukin-6
- IL-10:
-
Interleukin-10
- IT:
-
Intrathecal injection
- L-ASP:
-
L-Asparaginase
- LFS:
-
Leukemia-free survival
- MRD:
-
Minimal residual disease
- MTX:
-
Methotrexate
- OS:
-
Overall survival
- r/r B-ALL:
-
Refractory or relapsed B-cell acute lymphoblastic leukemia
- sCD25:
-
Soluble CD25
- TNF-α:
-
Tumor necrosis factor-α
- VDS:
-
Vindesine
References
Lazarus HM, Richards SM, Chopra R et al (2006) Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRCUKALL XII/ECOG E2993. Blood 108(2):465–472
Brown P, Inaba H, Annesley C, et al. NCCN clinical practice guidelines in oncology: pediatric acute lymphoblastic leukemia, version 1.2020. Accessed date: 30 May 2019.
Fielding AK, Richards SM, Chopra R et al (2007) Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG2993 study. Blood 109(3):944–950
Lee DW, Kochenderfer JN, Stetler-Stevenson M et al (2015) T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385(9967):517–528
Peter B, Hermann K, Gunter HR et al (2009) Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: The ALL-REZ BFM Study Group. J Clin Oncol 27(3):377–384
Shannon LM, Noelle F, Pamela AS et al (2014) Chimeric antigen receptor t cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517
He X, Xiao X, Qing L et al (2019) Anti-CD19 CAR-T as a feasible and safe treatment against central nervous system leukemia after intrathecal chemotherapy in adults with relapsed or refractory B-ALL. Leukemia 33(8):2102–2104
Dai H, Zhang W, Li X et al (2015) Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Onco Immunol 4(11):e1027469
Pullen J, Boyett J, Shuster J et al (1993) Extended triple intrathecal chemotherapy trial for prevention of CNS relapse in good-risk and poor-risk patients with B-progenitor acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Clin Oncol 11:839–849
Pan J, Niu Q, Deng B et al (2019) CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia 33(12):2854–2866
Pan J, Zuo S, Deng B et al (2020) Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood 135:387–391
Pan J, Yang JF, Deng BP et al (2017) High efficacy and safety of low dose CD19 directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 31(12):2587–2593
Kochenderfer JN, Wilson WH, Janik JE et al (2010) Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116(20):4099–4102
Maude SL, Frey N, Shaw PA et al (2014) Chimeric antigen recepatientor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517
Daniel WL, Bianca DS, Frederick LL et al (2019) ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25(4):625–638
Karschnia P, Jordan JT, Forst DA et al (2019) Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood 133(20):2212–2221
Matthew JF, Jorg D, Maria ML et al (2019) Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood 134(11):860–866
Nirav NS, Bryon DJ, Timothy SF et al (2020) Intrathecal chemotherapy for management of steroid-refractory CAR T-cell–associated neurotoxicity syndrome. Blood Adv 4(10):2119–2122
Zając SO, Pawlak MA, Karmelita KK et al (2020) Long-term brain status and cognitive impairment in children treated for high-risk acute lymphoblastic leukemia with and without allogeneic hematopoietic stem cell transplantation: a single-center study. Pediatr Blood Cancer 67(6):e28224
Bhojwani D, Sabin ND, Pei D et al (2014) Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol 32(9):949–959
Cheung YT, Sabin ND, Reddick WE et al (2016) Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of child-hood acute lymphoblastic leukaemia treated with chemotherapy: a longitudinal analysis. Lancet Haematol 3(10):456–466
Halsey C, Buck G, Richards S et al (2011) The impact of therapy for childhood acute lymphoblastic leukaemia on intelligence quotients; results of the risk-stratified randomized central nervous system treatment trial MRC UKALL XI. J Hematol Oncol 4:42
Gibson EM, Nagaraja S, Ocampo A et al (2019) Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 176(1–2):43-55.e13
Acknowledgements
We thank the patients and their families who participated in this study. And we also thank all physicians, nurse, and other patient care providers involved in the care of these patients.
Funding
This work was supported by the National Key R&D Program of China (2019YFA0110200), the National Natural Science Foundation of China (81870090), the Tianjin Science Funds for Distinguished Young Scholars (17JCJQJC45800) and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018PT32034).
Author information
Authors and Affiliations
Contributions
YT and JP contributed to data collection, data analyses, data interpretation. BD and AHC contributed to CAR T-cell manufacture. JP, ZL, WS, JX, JD and ZW contributed to clinical protocol. XY was responsible for leukemic cell immunophenotyping. YT and JP were responsible for all statistical analyses. YT, JP and XF wrote the manuscript. JP and XF directed the study and had final responsibility to submit for publication. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
AHC is also a founding member of Shanghai YaKe Biotechnology Ltd. The remaining authors declare no conflict of interest.
Ethical approval and consent to participate
The study protocols were approved by the institutional review board at Beijing Boren hospital, and the patients provided written informed consent. This clinical investigation was conducted according to the principles of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tan, Y., Pan, J., Deng, B. et al. Toxicity and effectiveness of CD19 CAR T therapy in children with high-burden central nervous system refractory B-ALL. Cancer Immunol Immunother 70, 1979–1993 (2021). https://doi.org/10.1007/s00262-020-02829-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02829-9